It’s no secret that individual plants within a species can vary in appearance—just peruse the range of Japanese maples (Acer palmatum) for sale at your local nursery. All belong to a single species, yet show diversity in traits like growth habit, foliage color, and leaf shape. It’s also old news that individuals can vary according to provenance (geographic source); winter hardiness is frequently noted as one of those variable physiological traits. Although he was not the first to note this phenomenon, botanist and plant explorer Joseph Hooker provided an early description in 1853. In an introductory essay preceding his notes on the flora of New Zealand, he described differences in the hardiness of Himalayan plants, “depending upon the altitude at which they were gathered.” Specifically, “some of the seedling Pines whose parents grew at 12,000 feet appear hardy, whilst those of the same species from 10,000 are tender. The common scarlet Rhododendron of Nepal and the North-west Himalaya is tender, but seedlings of the same species from Sikkim, whose parents grew at a greater elevation, have proved perfectly hardy.” A few years ago, we wrote about C. S. Sargent’s interest in acquiring cedar of Lebanon (Cedrus libani) germplasm that would prove to be hardy in Boston (Aiello and Dosmann 2007). He succeeded by obtaining seeds from Turkey, and those plants and others from that region have fared notably well in Philadelphia and Boston as well as colder climes, while accessions from other provenances have failed.
The cedar of Lebanon story points out the ongoing importance of plant exploration, a vital component of the missions of our respective arboreta. When adding accessions, we want to capture as much variation as possible within a species, so we often collect from multiple populations within a species’ range. This is standard practice for species in our core, or high-priority, collections that are already well adapted to our local Arboretum conditions. However, for species like C. libani that are not typically winter hardy in our climate, we must seek specific provenances that may hold hardier populations.
One of those marginally hardy species that has evaded our grasp so far is the southern live oak (Quercus virginiana), whose massive, gnarled form—often draped in Spanish moss (Tillandsia usneoides)—conjures up images of the antebellum South. This oak often exceeds 50 feet (15.2 meters) in height, but it is the spread that typically draws our attention. Almost always wider than tall, the colossal sweeping branches of old trees are a marvel. The common name “live oak” refers to the typically evergreen leaves, stiff and shiny on the top, and gray-tomentose on the bottom. However, during particularly cold spells the species may shed some of its leaves and is regarded as brevideciduous. Tolerant of drought as well as soil salinity and salt spray, southern live oak is often categorized as a “tough plant,” aside from winter hardiness issues.
The Quest Begins
In 140 years of acquiring and testing species from all over the temperate world, the Arnold Arboretum has never even attempted to grow Q. virginiana. That the Arboretum had tried—and failed—to establish hardy plants in the collection is one thing, but to never even try? That was a surprise. The situation was similar at the Morris Arboretum, where Q. virginiana acorns were received in the mid-1950s as part of the ambitious Michaux Quercetum project. Acorns from several collections germinated and were planted in the oak nursery, but none of these survived to be grown on because, “mortality during the first winter [in the nursery] was extremely high, and no trees survived the second winter” (Santamour 1960). With this history at both arboreta, we determined that it would be worth the effort to document and collect from trees that, like the special provenance of C. libani in Turkey, might be hardy for us in our respective regions.
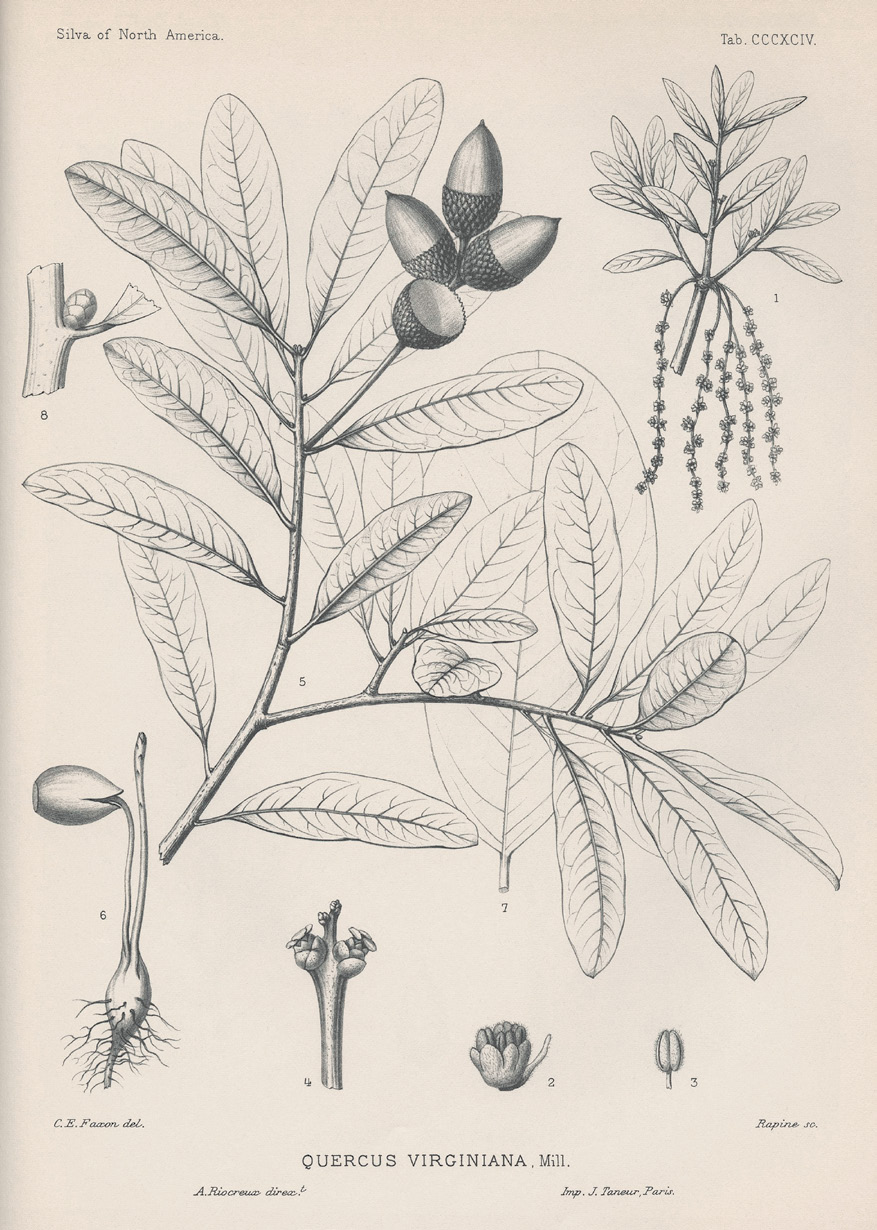

Southern live oak is native to the southeastern United States, with a range that extends from central Texas and a few populations in southwest Oklahoma, all along the Gulf Coast and Florida peninsula, turning northward to follow the coasts of Georgia, South Carolina, North Carolina, and southern Virginia. Flint (1997) noted that while the species’ useful range as a landscape plant is USDA Zone 8b (average annual minimum temperature 15 to 20°F [-9.4 to -6.7°C]), it can tolerate colder extremes like Zone 7b (average annual minimum temperature 5 to 10°F [-15 to -12.2°C]) but is unlikely to attain its full size and landscape value because of ice and snow damage. Recent research from the lab of Jeannine Cavender-Bares at the University of Minnesota has yielded interesting information on its ecology. Her lab found that Q. virginiana, like many other temperate species, varies in leaf and stem hardiness as a function of latitude: the more northern populations possess greater hardiness (Cavender-Bares 2007; Cavender-Bares et al. 2011; Koehler et al. 2012). In these studies, the lowest temperature that plants were exposed to (and survived) was 14°F (-10°C), which is still warmer than the average annual minimum temperatures found in Philadelphia (Zone 7a, 0 to 5°F [-17.8 to -15°C]) or Boston (Zone 6b, -5 to 0°F [-20.6 to -17.8°C]).
We feel there is potential to grow this species in our collections, or at least make the attempt. For one, our average annual minimum temperatures have risen because of climate change and urban heat island effects (see textbox). Although this hardly places us in the banana belt, it warrants an attempt to grow Q. virginiana. Also, the northernmost population sampled by Cavender-Bares was from Goose Creek State Park, North Carolina, where notably cold temperatures have occurred (down to 9°F [-12.8°C] in 1904). Surely if these populations survived that weather event, they likely possess greater hardiness than was indicated in experimental testing. Lastly, our review of various checklists, atlases, and other resources revealed that natural populations could be found around Norfolk and Virginia Beach, Virginia, (particularly First Landing State Park), as well as a few points northward—over 90 miles north of the Goose Creek sampling sites.



We wanted to collect germplasm from the most northerly natural populations in Virginia. Because some of these populations are near (or even within) urban areas, it is especially important to collect acorns and grow the seedlings elsewhere in case these populations become threatened by development in the future. During our planning, we also learned of notable trees that were either remnant natural populations or planted specimens that had survived frigid winters. These included old specimen trees growing in Hampton and Williamsburg (where it reached -7°F [-21.7°C] in 1985), and Richmond (-12°F [-24.4°C] in 1940). Even if these trees were planted (and therefore did not represent a wild source), their potential hardiness makes them valuable. And for a few of them, their extreme age suggests they were derived from now-extirpated local populations.
To Richmond
Our short trip (October 20th to 24th, 2012) to explore the Eastern Shore of Virginia started in Richmond and finished in Virginia Beach. Our first collection site was the campus of the University of Richmond, home of the Spiders. Upon arrival, we were impressed by the well groomed landscape, despite having hosted a football game the day before (they beat James Madison University, 35 to 29). We commented that either the students were notably well behaved, or the landscape services department worked through the evening hours.
Using directions provided by Professor of Biology John Hayden, we were able to easily find the various specimens, many of which had been planted in the last few decades. Although we had seen the occasional Q. virginiana before, this site gave us our first chance to really observe the species in depth. Our first two collections were from trees growing near Westhampton Lake. The first tree, rounded and spreading in form, was about 15 feet (4.6 meters) tall and twice as wide; we estimated that it had been growing in that location for 10 to 15 years. And it was loaded with acorns, most with bright yellowish green nuts and tawny brown caps. However, a few had started to turn the typical mature color, a rich burgundy-brown. The branches were dense, with short internodes, and thickly set with leathery, oblong to oval leaves. Considering their form and (brevi)evergreenness, we thought they would make great screens. As was our protocol for the entire trip, we gathered germplasm in the form of acorns, made herbarium vouchers from cut twigs (complete with the acorns), and of course jotted down copious collection details that pertained to the trees as well as the local conditions and environment. The second collection was from a nearby tree, smaller and younger than the first, but similar to another six growing nearby. Undoubtedly the campus was trying to establish a grove of these trees in this area. Before leaving the University, we located and collected from two trees, older than the first, which were growing near a dining hall.

Our next destination—after an amazing lunch at Buz and Ned’s BBQ—was Bryan Park, a historic Richmond landscape founded in 1910. We expected to find small, rounded trees similar to those we had found at the University earlier in the morning. However, what we did find were three very large individuals, just down the hill from the Gatekeeper’s House on the park’s northeast side. Heights ranged from 30 to 40 feet (9.1 to 12.2 meters); each was rounded, usually twice as wide as tall, and with gnarled, twisting stems and branches. Only two of the trees (with dbh values of 35 and 39 inches [89 and 99 centimeters], respectively) bore acorns. Although we do not have any records to confirm this, based on their size we assume that the trees date back to the founding of Bryan Park and approach the 100 year mark. If so, they certainly would have survived the frigid winter of 1940.
To Williamsburg
We departed Richmond in the early morning of October 22nd, and by 9:00 a.m. arrived at our next destination: the College of William and Mary in Williamsburg. Beth Chambers, curator of William and Mary’s herbarium, was a great help to our efforts. Prior to our arrival, she scouted the numerous southern live oaks on campus, and even collected a few acorns in case there were none to be had by the time we arrived. She also accompanied us during collecting, providing assistance as well as anecdotes about the trees and buildings of this historic campus and neighboring colonial village. There were numerous southern live oaks planted on the campus, and their history dates to even before the founding of the university in 1693. The Corner Live Oak, a famous tree on campus, had served as a prominent boundary marker until its removal in 1943. Its age was estimated to be about 300 years at that time. Prior to its removal, acorns were collected and the progeny Two mature southern live oaks east of the Wren Building on the College of William and Mary campus. were planted around campus, including a prominent line along Landrum Drive (Mathes 1992).

The southern live oak legacy is also preserved in an 1836 watercolor of the Wren Building, a prominent campus edifice named after the famous architect Sir Christopher Wren, who may have designed it. When we arrived at the Wren Building, we were greeted by a towering Q. virginiana on the southeast corner. Although it had few accessible acorns, just to the east were several other large trees, the tallest nearly 40 feet (12.2 meters) in height. We collected seeds and vouchers from three of these specimens, two of which appear in a photograph from about 1875 (http://www.history.org/foundation/journal/Winter11/old_williamsburg/#3). A number of trees also grew off campus, in the Colonial Williamsburg section of town. We made two additional collections from these town trees, and also made the interesting discovery of the Compton oak, Quercus × comptoniae, a hybrid between Q. virginiana and Q. lyrata (overcup oak). We ascertained its identity from Terry Thon, a basket maker for Colonial Williamsburg, who has been routinely collecting acorns from it for years. This tree was an impressive specimen with a dbh of 60 inches (152.4 centimeters) and a spread of 100 feet (30.5 meters), and we were anxious to make a collection, too. [Editor’s note: We’ll have more on the Compton oak in a future issue of Arnoldia.]
The Oaks of Fort Monroe
During the trip’s planning stage, Michael Dosmann spoke to Christopher Beagan of the National Park Service’s (NPS) Olmsted Center for Landscape Preservation. Christopher described the amazing oaks of Fort Monroe and insisted that we visit this population and others near Hampton. He shared a few photos of the trees and we were instantly interested. He put us in touch with one of his NPS colleagues, Eola Dance, who is the Chief of Visitor Services and Resources Management at the Fort. We were thankful for the lead.
Perched at the ocean’s edge, the Fort has a rich history that dates to the early seventeenth century. It had been occupied by the military until its recent decommissioning in 2011, and it is now a National Monument. The massive six-sided stone structure is the largest of its kind in North America: 63 acres of land surrounded by walls and an impressive moat. Construction of the current Fort took 15 years to complete and the final phase (finished in 1843) was overseen by Robert E. Lee. In an ironic twist, such was its fortitude that it was never lost to the Confederacy.




We arrived in the late afternoon of the 22nd to meet Eola, who enthusiastically showed us around the facility and explained some of its fascinating history. We also returned on the morning of the 24th to visit with her, as well as Joshua Gillespie and Robert Kelly of the Fort Monroe Authority. Inside the buttressed edifice we found a composite of former army barracks, period officer quarters, office and training facilities, storage buildings, a chapel, and a museum, as well as nearly 350 southern live oak specimens scattered throughout. Perhaps the most impressive is a large grove that grows along the south and west edge of the interior parade ground. Some trees stood as lone sentries, while others grew in small groups, sometimes arching over the sidewalks and defying gravity. Most were no taller than 35 to 40 feet (10.7 to 12.2 meters), and all had dramatic, ethereal forms, the result of decades and even centuries of difficult environmental conditions including drought, intense heat, and salt spray (even inside the fort’s walls). No doubt, the grandest of these was the Algernourne Oak, a leviathan estimated to be over 450 years old. This tree has a basal diameter of 90 inches (228.6 centimeters), with two massive leaders diverging about 3 feet (0.9 meters) above the ground. True to the species’ form, the tree’s height is around 60 feet (18.3 meters), but its spread is nearly 100 feet (30.5 meters).
The acorns on all the oaks at Fort Monroe were few and far between, so we collected only herbarium vouchers from this representative population. We assumed that these trees produced few acorns because of the exposed, hot and dry location, and the droughty summer. That same exposed and hot nature of the fort is probably the reason these trees still exist. People needed shade, and because few other trees were capable of growing in such an environment, this remnant natural population was left in place and even allowed to regenerate (perhaps with a bit of assistance from the local inhabitants). Standing in the parade ground, we imagined ourselves dressed in full uniform, performing drills and marching for hours under the hot sun and dry, salty breeze—those trees would be considered sacred! The trees were in remarkably good condition considering their age, size, and the heavy impact of human activities on the site. Many of them showed the marks of time but they were mostly healthy and growing well, a testament to the resilience of southern live oaks.
First Landing
We dedicated the 23rd to surveying the flora of First Landing State Park, which lies on Cape Henry between Norfolk and Virginia Beach. Its current name, changed from Seashore State Park in 1997, acknowledges this site as the location where the Virginia Company first landed in 1607 prior to settling Jamestown. The park covers about 3,000 acres, and comprises eight upland plant community types that range from dune crests to mesic forests (Clampitt 1991). Our initial foray was into the mesic forests where several of our non-oak collecting targets were to be found: devilwood (Osmanthus americanus) and swamp bay (Persea palustris). Like southern live oak, these two species of shrubs or small trees are near or at their northernmost ranges in Virginia. And, for reasons similar to our quest for hardy southern live oak germplasm, we were anxious to locate and collect from these species.
Finding them was quite easy thanks to our earlier planning conversations with Erik Molleen of the Virginia Department of Conservation and Recreation; the fact that there was an Osmanthus Trail in the park was also helpful. Osmanthus americanus specimens were numerous and scattered throughout the understory. They became easy to identify from a distance because their glossy green leaves are arranged oppositely, as with other members of the olive family (Oleaceae). At the Arnold Arboretum, this species has proven to be quite a challenge to cultivate because of cold hardiness issues. One clone, a cultivated lineage from Spring Grove Cemetery in Cincinnati, Ohio, has been reliably hardy in Boston. Likewise a plant at the Morris Arboretum has survived but not thrived since it was received from a local nursery in 1962. Wild-provenance material has long been a target because of the species’ botanical and ornamental appeal. Its broadleaved evergreen foliage provides winter interest, and the small, creamy white flowers in spring are a delight to the nose; their mellic scent beckons from great distances. We were able to collect fruits—bright green drupes at this stage—from many trees in the woodland.




Persea palustris also dotted the understory, and, like devilwood, has large, elliptic, evergreen leaves. However, the leaves are coarser in texture and borne alternately in this member of the laurel family (Lauraceae), and the fruits (also drupes) were an eye-catching purplish blue at this stage. With only a bit of imagination, it is easy to see the kinship to Persea americana, the avocado. However, with drupes less than ½ inch long, they wouldn’t yield much guacamole.
Many other plant species caught our eyes. Sand hickory (Carya pallida) grew in and along the higher ridges. This species was also on our target list, but there were very few fruits to be found; those we did stumble upon were on the ground and of poor quality. While scouring the ground, it was a treat to see Indian pipe (Monotropa uniflora), the nodding white flowers and stems appearing like dancing apparitions among the pine cones. Looking up, we noticed many leaves of sourwood (Oxydendrum arboreum) at their peak for autumn color, the brilliant reds and oranges echoed in the near-spent needles of bald cypress (Taxodium distichum). These bald cypress trees were impressive, conjuring up images of great swamps, and yet we were only a few hundred meters from sand dunes and the ocean (a reminder of how quickly landscapes change). Because the water level was down considerably, their buttressed trunks and knees were exposed to reveal an amazing network of lignified stalagmites. Throughout the woodland landscape, Spanish moss draped across the limbs and branches like overloaded Christmas tree tinsel. As with southern live oak and devilwood, southeast Virginia marks the northern edge of the native range for this rootless member of the pineapple family (Bromeliaceae).



After a brief lunch, we explored the shoreline of First Landing, a strip considerably different than what we saw in the morning. The morning site was lush and diverse, but this sandy strand was quite the opposite. Oaks—primarily southern live oak plus some bluejack oak (Q. incana)—dominated this landscape to create a band of dense vegetation that was pruned by the salt-laden winds into interesting forms and habits. As we had seen with the cultivated plants, the live oak trees were wider than tall (but rarely over 20 feet [6.1 meters] in height) and frequently had multiple stems and a low-branching form. One of the larger trees we found had three stems measuring 12.5, 17, and 21 inches (31.8, 43.2, and 53.3 centimeters) in diameter at 12 inches (30.5 centimeters) above the ground. Despite the stressful environment, trees were healthy and there was noticeable regeneration of young seedlings in the understory, which is always a good sign. Rather than focus on individual trees at this site, we maximized the amount of genetic variation in the collection by gathering acorns from 12 trees. Some trees were so fecund and at perfect ripeness that we could easily shake the branch and scores of the nuts would drop from their caps.
Next Steps
Although the fieldwork is complete, the data are in the databases, and the herbarium specimens are mounted, much work remains ahead of us. Each of our institutions is hard at work germinating the seeds from the various collections made on the trip—twelve separate Q. virginiana collections, plus one each of the Persea, Osmanthus, and Q. × comptoniae. We plan to try several different methods to successfully coax the oaks into cultivation. For starters, we captured a wide swath of variation during our trip—one never knows just which germinating seedlings from which populations will be the ones to survive. Because young plants are less cold hardy than older ones, we plan to hold some seedlings in containers for a few years before planting them into nurseries. And, because each of our arboreta has microclimates that are warmer than our nursery areas, we also plan to plant some young plants directly into those microclimates, skipping the nursery altogether. For marginal species such as these, success often is achieved by those who hedge their bets.
Sidebar | Changes in Plant Hardiness Zones
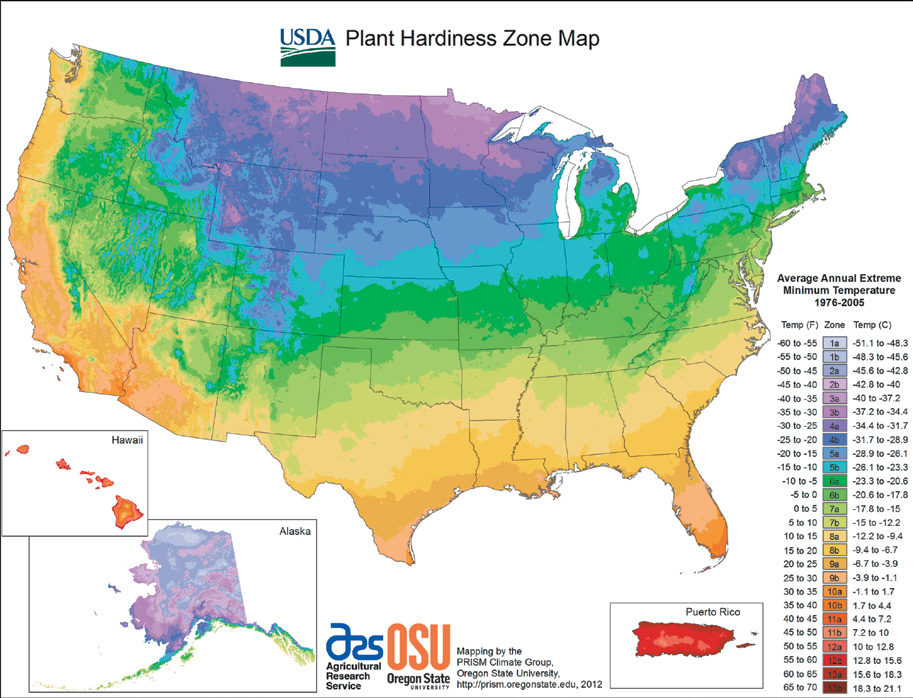
In January 2012, the United States Department of Agriculture unveiled its new Plant Hardiness Zone Map (PHZM) (http://planthardiness.ars.usda.gov/PHZMWeb/), a development that was long anticipated by gardeners and researchers. Like its earlier incarnations, the new PHZM provides guidelines to predict a region’s average annual minimum temperature, a vital statistic in determining whether or not a plant may survive the winter in a particular area. Last updated in 1990, the map now features a number of significant features. For one, it has gained interactivity through a Geographic Information System (GIS) that enables users to zoom in at regional and state levels; it also has a tool to identify a zone by zip code. Data quantity and quality represent marked improvement in the map’s reliability—the new PHZM utilizes 30 years (1976–2005) and a wider geographic sampling of weather station data. (In comparison, the 1990 PHZM used data from only a 13-year period, 1974–1986, and fewer stations.)
Compared with the 1990 version, zone boundaries in the new edition have shifted in many areas, typically about a half-zone warmer from their previous designation (although some have shifted to a colder zone). Some of the changes are the result of the new, more sophisticated mapping methods and greater numbers of station observations, which has greatly improved accuracy, especially in mountainous regions. Additionally, in urban and suburban regions, the cities themselves can greatly influence temperature, resulting in heat islands that make them significantly warmer than their rural surroundings.
The data solidify the reality of climate change, suggesting even greater unpredictability with regard to future weather patterns and environmental conditions. The implications are significant not just for the natural world and those who study it, but also for gardeners. Warmer temperatures in the colder months can lead to further pest and disease outbreaks, as both are better able to survive in mild winters. Plants at the southern limits of their adaptability may eventually be negatively impacted to the point where they are useful solely at more northern sites.
On the positive side, warmer zones allow for an expanded palette of plants that gardeners can reliably grow. For instance, in Philadelphia there is now a better chance of growing traditional southern favorites such as crape myrtle (Lagerstroemia spp.), southern magnolia (Magnolia grandiflora), and Japanese camellia (Camellia japonica). In New England, the change in hardiness may allow gardeners to reliably grow Stachyurus praecox and Chimonanthus praecox, which are currently hardy only in protected microclimates. And, if we are lucky, Philadelphia and Boston can add Quercus virginiana to that list.
Literature Cited
Aiello, A. S. and M. S. Dosmann. 2007. The quest for the hardy cedar-of-Lebanon. Arnoldia 65(1): 26–35.
Cavender-Bares, J. 2007. Chilling and freezing stress in live oaks Quercus section Virentes: intra- and inter-specific variation in PS II sensitivity corresponds to latitude of origin. Photosynthesis Research 94: 437–453.
Cavender-Bares, J., A. Gonzalez-Rodriguez, A. Pahlich, K. Koehler, N. Deacon. 2011. Phylogeography and climatic niche evolution in live oaks (Quercus series Virentes) from the tropics to the temperate zone. Journal of Biogeography 38: 962–981.
Clampitt, C. A. 1991. The upland plant communities of Seashore State Park, Virginia Beach, Virgina. Virginia Journal of Science 42: 419–436.
Flint, H. L. 1997. Landscape plants for eastern North America. New York: John Wiley and Sons, Inc.
Hooker, J. D. 1853. The botany of the Antarctic voyage of H.M. discovery ships Erebus and Terror in the Years 1839–1843, under the command of Captain Sir James Clark Ross. London: Reeve Brothers.
Citation: Dosmann, M. S. and Aiello, A. S. 2013. The quest for the hardy southern live oak. Arnoldia, 70(3): 12–24.
Koehler, K., A. Center, J. Cavender-Bares. 2012. Evidence for a freezing tolerance–growth rate trade-off in the live oaks (Quercus series Virentes) across the tropical–temperate divide. New Phytologist 193: 730–744.
Mathes, M. C. 1992. The Planting of a Campus Tradition: A History of the Landscape of the College of William and Mary (revised edition). Williamsburg, Virginia: College of William and Mary.
Santamour, F. S., Jr. 1960. Western and southern oaks in the Michaux Quercetum. Morris Arboretum Bulletin 11(1): 7–10.
Michael S. Dosmann is Curator of Living Collections at the Arnold Arboretum and Anthony S. Aiello is the Gayle E. Maloney Director of Horticulture and Curator at the Morris Arboretum of the University of Pennsylvania in Philadelphia.
From “free” to “friend”…
Established in 1911 as the Bulletin of Popular Information, Arnoldia has long been a definitive forum for conversations about temperate woody plants and their landscapes. In 2022, we rolled out a new vision for the magazine as a vigorous forum for tales of plant exploration, behind-the-scenes glimpses of botanical research, and deep dives into the history of gardens, landscapes, and science. The new Arnoldia includes poetry, visual art, and literary essays, following the human imagination wherever it entangles with trees.
It takes resources to gather and nurture these new voices, and we depend on the support of our member-subscribers to make it possible. But membership means more: by becoming a member of the Arnold Arboretum, you help to keep our collection vibrant and our research and educational mission active. Through the pages of Arnoldia, you can take part in the life of this free-to-all landscape whether you live next door or an ocean away.