I
September is the attenuated tail of summer. The last flowers of great blue lobelia bloom in meadow openings or in partially shaded forest edges where they can find a little extra soil moisture. Tangles of calico aster spill into the trails, branching and short-leaved, strewn with flowers. White rattlesnakeroot flowers hang like trombone bells at the ready. Jack-in-the-pulpit berries turn gradually from green to red, the masses of fruits on some plants as variable as kernels of multicolored flint corn. False Solomon’s seal berries ripen from salmon to bright red and become thin-skinned and heavy with juice. Blue cohosh seeds ripen on the plant, toxic but delicious looking, a rich blue that will hold its own through winter, when you may still find an occasional seed abandoned by a gray squirrel on top of a fallen log, beside a scattering of scratched red oak acorn shells.
Acorn production peaks in northern Illinois around the first of the month. Nuts pile up along the trails. Many are immediately split open by squirrels or eaten by deer. Others are not eaten by mammals but are preyed upon by weevils that devour the starchy cotyledons and fill the shells with frass, exposing the baby plants to fungi and desiccation. In some cases, the only violence weevils do the seedling is to deprive it of some of the nutrients left by the mother tree in its cotyledons. Perhaps this will be enough to kill the seedling over time, leaving it too weak to hold on for a few years in the understory until there is a blowdown or an old tree falls, taking a few others with it, letting in enough light for the baby oak to photosynthesize on its own and possibly win the race to the canopy. If it fails to do so, the oak will never produce offspring of its own.
The seeds falling from the tips of the tree of life in the weeks flanking the equinox are the ones we will find growing next spring. They were sparked into life in an instant of unlikely pollination. They were provisioned with food all through development. Now, we find them dispersed on feathers or fur, in the stomach of a bird, in mud lodged between toes or talons or claws. Some are dropped unceremoniously at the base of the tree to roll downhill, in a move that might appear clumsy, but what are appearances? Each species has gambled successfully over tens of thousands of generations, if not more, on that drop to the ground or that risky flight on wind, or on the passage of squirrels or jays or extinct passenger pigeons or mammoths whose interests were never identical to those of the trees. The dice drop; then the plant prepares for winter. Perhaps their seeds will germinate before they can be eaten by a vole or squirrel. If so, these notes of fall will echo for hundreds of years.
II
Near the end of September, the white fungal bodies of aborted entoloma sprout from the leaf litter like manna. These knots of mycelia push leaves out of the way overnight to sit on the surface of the forest floor. Over a few days, they grow into misshapen white loaves as large as an infant’s fist. They are caused by a pathogenic fungus (Entoloma abortivum)1 infecting one of the honey mushrooms (Armillaria gallica).2 The latter are known best from their black, cord-like rhizomorphs that scout the soil’s surface for trees to infect and then ascend the trunks beneath the bark, where they remain long after the tree is dead. Near these masses of intertwined Armillaria and Entoloma mycelia, the yellowish-brown fruiting caps of Armillaria can often be found, and sometimes the whitish caps of Entoloma as well. By night, when the rains have been just right, glowing Armillaria marks the edges of the trails, ghosts of the cambium devoured by the fungal mycelia. Rings of light mark the ends of severed boles, squeezing through passages where sunlight formerly passed from the leaves down to the roots as fixed sugars.
Stump puffballs (Apioperdon pyriforme) sprout from downed logs or form colonies in the wood chips. Then their insides turn to spores. The precocious ones desiccate and become brown inside while their peers are still white and fleshy or just turning granular inside the taut skin. Chicken of the woods (Laetiporus sulphureus) sprouts from standing dead ashes, fallen oaks, and rotting trees of several species, forming scalloped orange shelves of delicious flesh. Months hence, its bleached carcasses will mark where the fungal bodies clung bright as lanterns to the dead trunks. Chanterelles (Cantharellus sp.), bonnets (Mycena sp.), oyster mushrooms (Pleurotus sp.), and giant puffballs (Calvatia sp.) emerge and then dry or decompose over the course of a few autumn weeks.
As these decomposers crowd the woods, the flowering plants become increasingly tattered. Jewelweed spanned the entire growing season, beginning as forests of nickel-sized cotyledons crowded under the leaf litter in late March and then rising into rolling hills of adult plants that dominate the landscape well into September.3 Now, it begins to yellow and wilt, thinning and breaking over. Wild ginger leaves glow with golden margins as they senesce, like autumn leaves of Ginkgo biloba. False Solomon’s seal becomes variegated and stringy, the vessels running the length of its leaves draped with torn and yellowing epidermis. Sheaths surrounding the glistening black wild leek seeds split open. The seeds stare out at the coming winter for a few days before they drop to the ground. Hop sedge and Gray’s sedge become decrepit, and the swollen skins of their perigynia disintegrate. Straight-stigma and curly-stigma wood sedges shatter, scattering their last achenes onto the bare soil.
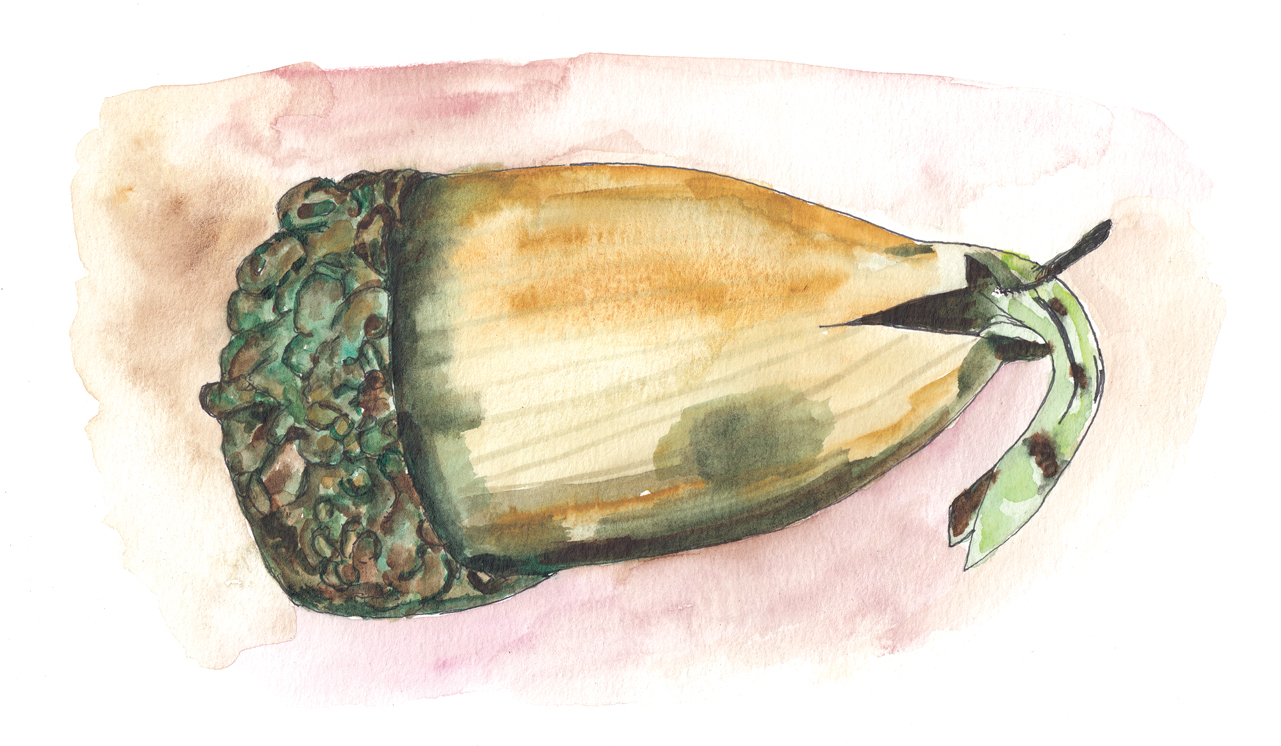
The white oak acorns that have made it this far lie half embedded in the soil. They split at one end, opposite the cap, cracked open by the emerging root that swells in the autumn rains. They are feeling their way blindfolded, trying to get a toehold while there is still time. Their impulse to grow is strong: collect a bagful of acorns, toss it into the refrigerator next to the carrots, and keep it cool and moist and dark; even there, some will start to germinate, senselessly looking for soil. The katydids have become quiet, and the morning-time crickets purr. They and the acorns are pacing the autumn to and fro,4 getting a little work done in advance of spring.
III
Rain falls and temperatures fluctuate in early October. Fog pools in the prairies beneath the power lines and drapes between the spruces. The trails become sodden. Stump puffballs ripen on fallen logs or stumps, syrupy brown. Earth stars (Geastrum sp.) crank their wings out and grip the soil. Young stinkhorns (Phallus sp.) erupt, crowded together like brussels sprouts, crawling with stink bugs. Over the course of several days, they grow obscenely to several inches in height and swarm with gnats. A few days later, they become flaccid and rot.
One morning last year, our woods at the Morton Arboretum were overrun by spring peepers. I started hearing their squeaks, trills, and whistles on my walk into work, their sounds shifted upslope from the wetlands where they had been calling six months earlier. There wasn’t anything they could be except for spring peepers. I did not expect them, however, and I consequently could not convince myself at first that I was hearing correctly. Songbirds were migrating, and I told myself the calls I heard were those of some itinerant bird I didn’t recognize. I waded into the sunflowers and towering wild lettuce to flush out any birds that might be there, but the calls stopped, as frog calls always do when you go hunting their source. They picked up again after I was safely back on the trail. After about ten minutes of this, it was clear I was hearing frogs. Peeps punctuated the woods west of Big Rock Visitor Station and all the way down to the service road that runs north through the meadow. I walked into work surrounded by them.

The peepers were with us for at least a few days. Colleagues found them in leaf traps and reported hearing their songs throughout the woods at all times of the day. Chorus frogs had also rediscovered their voices and were trilling in the warm afternoons. On a cool morning midweek, I made a quick stop to listen for the spring peepers again. The forest was silent. Gnats buzzed around the stinkhorns. Then, from a hollow tucked between the shortcut trail to Big Rock and the trail that runs west along the ridge of the moraine, a single peeper called. I walked down into the hollow and poked around for five or ten minutes, but there were no other calls.
In early October, sugar maple leaves are turning yellow and starting to fall. Lower branches of the American black elderberry corymbs are broken, and ray flowers fall from wingstem in the floodplains. Zig-zag goldenrod heads are pale with feathery achenes. Wood nettle leaves are chewed to lace but still have plenty of sting left. Bedraggled pale jewelweed provisions its late-season capsules, galls blistering along its leaf midveins and darkening along one side. Wild leek has dropped about a third of its seeds. Fowl mannagrass culms are reclining. Enchanter’s nightshade leaves have almost all fallen, leaving the stalks bristling with fruits. But false rue anemone, one of our iconic spring ephemerals, often begins sending up fresh shoots. The species is known to be a fall germinator,5 a rarity in our forests. Yet many people miss it in the fall,6 myself included for my first twenty-five years as a naturalist.
By mid-October, white-throated sparrows pass through town on their way southward and fill the fields with “tssts” and whistles, marauding the shrubs for berries and insects. Near sunrise, a single bird may belt out its spring song, the bold three-toned “Old-Sam-Peabody-Peabody-Peabody” or the two-toned “Oh-Canada-Canada-Canada.”7 The territorial song sparrows join in as they are skipping town, possibly defending their territory on the way south,8 as they did on the way to their breeding grounds in the north.
These discordant echoes of spring reverberate through the months of fall: frog calls, spring wildflowers emerging under the year’s falling leaves, sparrows guarding territory as though it were breeding season. Signs of the changing season are deeply inscribed, paid for with the lives of individuals whose instincts weren’t as well tuned. Time your emergence right, and you’ll make it through winter. Time it wrong, and you may not. A million hard-earned habits comprise this business of laying up treasure on earth, where the moth and dust corrupt. These are the forest’s strategies for getting through winter. Beauty is a byproduct.
IV
Chlorophyll molecules become unhooked from the proteins that bind them as the days shorten and the nights become colder. They become phototoxic to the leaves in which they reside. Each leaf then begins the process of autumn housekeeping, breaking the chlorophyll into harmless components that can be recycled.9 It reabsorbs nitrogen, nutrients, and basic elements that are costlier to assimilate than to recycle. As the engines of photosynthesis are disassembled and reabsorbed, carotenoids are exposed, producing the brilliant yellows of fall. Anthocyanin production picks up, producing reds and oranges that may protect the leaf from sun or insects for a few weeks.10 It is a short period of intense color, shaped by the balance of daytime and nighttime temperatures, the shortening hours of daylight, the timing of precipitation, and the internal coordination of chlorophyll degradation, redistribution of resources in the tree, and the production of new pigments. Activity at the molecular level scales up to cells, to leaves, to canopies, finally to hillsides in color.
Last year, an early snow fell on Halloween, weighing tree branches down and tearing leaves off prematurely. The next morning, an hour after sunrise, yellow sugar maple leaves chirped almost inaudibly as they hit the fresh snow and glowed like lanterns on its surface. The skeletons of jewelweed were knocked to the ground. The wood nettle leaves, frozen, hung like rags. A woodcock stopped over on its way south, skating past a twelve-inch diameter red oak that had been hauled down by the snow. Fall can be over in a moment.
In most years, though, autumn funnels down to winter. Conduits between tree leaves and their branches are squeezed by a scar forming at the base of the petiole, and the trees rain resources. Leaves falling to the soil return calcium, nitrogen, and other nutrients that were shuttled upward all through the summer. Maples, basswoods, ashes, tulip trees, sassafras, and black cherries shed nutrient-rich leaves that are thin and tasty. These decompose rapidly, forming an ephemeral and semitranslucent sheet over the soil’s surface. Oaks, American beeches, and shagbark hickories drop leaves that decompose more slowly, remaining on the forest floor where they insulate and provide the raw material for rodent and insect activity and the matrix for ground fires.11 The chemical composition of these leaves, particularly their calcium content, shapes the sounds we will hear the next year in the quiet evenings, as Eurasian earthworms drag whole leaves into their burrows, selecting the calcium-rich species first12 and shushing along under the leaf litter. When I stamp my foot next summer, shaking the ground, the earthworms will all slurp down into their burrows and go silent for a moment before they begin again: shh, shh, shh.
June beetle grubs go dormant. Cicada children, patient by nature, gradually cease their subterranean feeding. Forest understory herbs move their resources back into their corms or bulbs or rhizomes or bequeath them to the forest floor. Bald-faced hornet queens crawl into rotting logs and prepare for winter, quiet and still. Rotten black walnut husks disintegrate in puddles at the bases of hills. Needle ice appears again in the wet soil.
V
By the end of November, the days are cool and overcast. White oak and red oak and sugar maple leaves interbed. A few seedlings continue twisting over soggy earthworm castings that erode to granules beneath the litter. The crickets and birds are quiet, and colors become subdued. People are mostly gone from the woods. Orion begins to show up in the evening sky, gliding upward from the eastern horizon just about the time we are settling into bed.

The musclewood, ironwood, beeches, and oaks rattle with marcescent leaves: the branches either mistimed or willfully ignored the last freeze of the year, and in so doing, they failed to produce the scars that would have severed these leaves from the tree.13 The squirrels have mostly gleaned the acorns and walnuts they need. Their messy nests are exposed in the treetops. Bark on many of the slender ashes and sugar maples throughout the woods is shredded where bucks have rubbed, scraping the velvet from their antlers. Jewelweed skeletons are broken over and knocked to the ground. Zig-zag and elm-leaved goldenrods are sparsely fuzzed with achenes, while white snakeroot has fully dispersed its fruits, and the few remaining bracts that once subtended the flowerheads are recoiled and twisted like starfish arms. Sandhill cranes fly southward in flocks of a hundred or more, their backs scraping the clouds.
Snow comes and goes, piling up on turkeytail fungus (Trametes versicolor) and secluding itself in the bark fissures of fallen logs. Juncos and chickadees glean and then spread the persistent berries of honeysuckle and gray dogwood. They are setting next year’s seedlings into motion. White avens, spinulose wood fern, hepatica, white bear sedge, and a handful of other common species photosynthesize beneath the falling snow. The rhizomes of spring wildflowers are suspended for a moment, appear to rest for winter, but extend by a hair’s breadth each time the soil thaws, bending around a buried stone. The future slowly unrolls with each cell division, shaping the forest we’ll walk through two and three springs hence.
Leaves abscise at intervals. They gyre downward. They touch the ground. Then, there is the shush of leaves against leaves. Everything that falls accumulates and shapes the forest floor. Here, a falling tree hides the entrance to a mouse’s home and crushes a mass of puffballs, and spores are dispersed. Over there, the leaves pile deeply, and then a windstorm blows them away so that the next year’s fires will not burn through: as a consequence, a handful of sugar maple seedlings survives one more year in the understory. These are the endings that form the forest’s beginning.
Plants Referenced
| Acer saccharum | Sugar maple |
| Ageratina altissima | White snakeroot |
| Allium tricoccum | Wild leek; A. burdickii is sometimes recognized as a distinct species, and my account also applies to that species |
| Arisaema triphyllum | Jack-in-the-pulpit |
| Asarum canadense | Wild ginger |
| Carex albursina | White bear sedge |
| Carex grayi | Gray’s sedge |
| Carex lupulina | Hop sedge |
| Carex radiata | Straight-stigma wood sedge (I made up this common name, because the sometimes-applied “straight-styled wood sedge” is a misnomer; the stigmas separate this species from C. rosea, not the styles) |
| Carex rosea | Curly-stigma wood sedge |
| Carpinus caroliniana | Musclewood |
| Carya ovata | Shagbark hickory |
| Caulophyllum thalictroides | Blue cohosh |
| Circaea canadensis | Enchanters’ nightshade |
| Cornus racemosa | Gray dogwood |
| Dryopteris carthusiana | Spinulose wood fern |
| Enemion biternatum | False rue anemone |
| Fagus grandifolia | American beech |
| Fraxinus sp. | Ash |
| Geum canadense | White avens |
| Glyceria striata | Fowl mannagrass |
| Helianthus sp. | Sunflowers; here the common woodland species are H. strumosus and H. decapetalus |
| Hepatica sp. | Hepatica |
| Impatiens pallida | Pale jewelweed; the description in the first half of this essay also applies to I. capensis, though I. pallida is the more common in the woods I frequent |
| Juglans nigra | Black walnut |
| Lactuca sp. | Wild lettuces |
| Laportea canadensis | Wood nettle |
| Liriodendron tulipifera | Tulip tree |
| Lobelia siphilitica | Great blue lobelia |
| Lonicera sp. | Honeysuckle |
| Maianthemum racemosum | False Solomon’s seal |
| Nabalus albus | White rattlesnakeroot |
| Ostrya virginiana | Ironwood |
| Prunus serotina | Black cherry |
| Quercus alba | White oak |
| Quercus rubra | Red oak |
| Sambucus canadensis | American black elderberry |
| Sassafras albidum | Sassafras |
| Solidago flexicaulis | Zig-zag goldenrod |
| Solidago ulmifolia | Elm-leaved goldenrod |
| Symphyotrichum lateriflorum | Calico aster |
| Tilia americana | Basswood |
| Verbesina alternifolia | Wingstem |
Endnotes
1 Czederpiltz, D. L. L., Volk, T. J., and Burdsall, Jr., H. H. 2001. Field observations and inoculation experiments to determine the nature of the carpophoroids associated with Entoloma abortivum and Armillaria. Mycologia, 93: 841–851. And for a readable summary: Volk, T. J. 2006. Entoloma abortivum, the aborting Entoloma, a.k.a. hunter’s heart, totlcoxcatl, or “ground prunes.” University of Wisconsin Plant Teaching Collection. Retrieved from: http://botit.botany.wisc.edu/toms_fungi/sep2006.html.
2 For a great article on the story of Armillaria taxonomy: Volk, T. J. 2002. The humongous fungus—Ten years later. University of Wisconsin Plant Teaching Collection. Retrieved from: http://botit.botany.wisc.edu/toms_fungi/apr2002.html.
3 The renowned forest ecologist John T. Curtis wrote of jewelweed, “One interesting response to light is frequently seen in mesic forests in which selective logging has been practiced so that large openings have been made in the canopy. The yellow jewelweed (Impatiens pallida) regularly forms an almost pure stand under such openings. This succulent and tender annual is very sensitive to light and is markedly reduced in height at diminished intensities. The colonies thus take on the characteristics of an integrating light meter, with the tallest plants in the center of the colony and shorter and shorter plants toward the edges. They produce contoured mounds which reflect the chance peculiarities in shape of the canopy opening with surprising accuracy.” Curtis, J. T. 1959. The Vegetation of Wisconsin: An Ordination of Plant Communities (pp. 122–123). Madison: University of Wisconsin Press.
4 “Listen to the rain, more rain, treadling earth to the sodden cold wet spun heads of this room, pacing the winter to and fro.” Borodale, S. 2012. 3rd December: Notes. Bee Journal. London: Jonathan Cape.
5 Baskin, J. M., and Baskin, C. C. 1986. Germination ecophysiology of the mesic deciduous forest herb Isopyrum biternatum. Botanical Gazette, 147: 152–155.
6 I have only dipped my toe into the woodland phenology literature, but an unpublished report by Max Partch is an interesting example. Partch took pains to observe all plant phases across numerous species well into October and still included no observations of new growth in the fall. Partch M. 1999. Plant phenology in central Minnesota. Biology Faculty Publications, 1. Retrieved from https://repository.stcloudstate.edu/biol_facpubs/1
7 If you sense that the white-throated sparrow song has changed over the past decade or so, you may not be imagining it. Since 2000, the two-noted song has spread across the breeding ground in Canada to largely supplant the three-noted song, perhaps due to tutoring in the wintering grounds. Otter, K. A., Mckenna, A., LaZerte, S. E., and Ramsay, S. M. 2020. Continent-wide shifts in song dialects of white-throated sparrows. Current Biology, 30: 3231–3235.e3
8 Wingfield, J. C., and Soma, K. K. 2002. Spring and autumn territoriality in song sparrows: Same behavior, different mechanisms? Integrative and Comparative Biology, 42: 11–20.
9 Christ, B., and Hörtensteiner, S. 2014. Mechanism and significance of chlorophyll breakdown. Journal of Plant Growth Regulation, 33: 4–20.
10 The potential adaptive role of leaf coloration is an area of active study. For an informative review, see: Archetti, M., Döring, T. F., Hagen, S. B., Hughes, N. M., Leather, S. R., Lee, D. W., Lev-Yadun, S., Manetas, Y., Ougham, H. J., and Schaberg, P. G., et al. 2009. Unravelling the evolution of autumn colours: An interdisciplinary approach. Trends in Ecology & Evolution, 24: 166–173. For a recent evaluation of competing hypotheses: Pena-Novas, I., Archetti, M. 2020. Biogeography and evidence for adaptive explanations of autumn colors. New Phytologist, 228(3): 809–813.
11 Among the species I have included here, those dominated by arbuscular mycorrhizae (e.g., the maples) tend to decompose more quickly than those by ectomycorrhizal fungi (e.g., the oaks). Phillips, R. P., Brzostek, E., and Midgley, M. G. 2013. The mycorrhizal-associated nutrient economy: A new framework for predicting carbon–nutrient couplings in temperate forests. New Phytologist, 199: 41–51.
12 Holdsworth, A. R., Frelich, L. E., and Reich, P. B. 2012. Leaf litter disappearance in earthworm-invaded northern hardwood forests: Role of tree species and the chemistry and diversity of litter. Ecosystems, 15: 913–926.
13 My understanding of this phenomenon comes primarily from an unpublished University of Wisconsin–Madison botany thesis on the anatomy of marcescent leaves in black oaks. As far as I know, the only published report on the thesis is a brief article I wrote in 1996 for NewsLeaf, the newsletter of the University of Wisconsin–Madison Arboretum, then updated in 2005 as “When Oak Leaves Fail to Fall,” Plant Health Care Report, 2005.03: 11–12; reprinted in 2007 in the Taltree Arboretum’s newsletter, Tag Along, 6: 6–7.
Andrew Hipp is the senior scientist in plant systematics and herbarium director at the Morton Arboretum in Lisle, Illinois. He conducts research on the origins and implications of plant diversity, with a focus on oaks, sedges, phylogenetic ecology, and trait evolution. You can read about his research at http://systematics.mortonarb.org and follow his natural history blog at https://botanistsfieldnotes.com.
Rachel Davis is an independent visual artist in the Chicago area. She works at the interface of natural science, abstract painting, printmaking, and textiles, integrating the formal and empirical elements of the natural world in her work. You can see more of her work at https://artbumble.com and follow her on Instagram: @art_bumble.
Citation: Hipp, A.L. And Davis, R. D. 2020. Each Year in the Forest: Autumn. Arnoldia, 78(2): 34–43.
From “free” to “friend”…
Established in 1911 as the Bulletin of Popular Information, Arnoldia has long been a definitive forum for conversations about temperate woody plants and their landscapes. In 2022, we rolled out a new vision for the magazine as a vigorous forum for tales of plant exploration, behind-the-scenes glimpses of botanical research, and deep dives into the history of gardens, landscapes, and science. The new Arnoldia includes poetry, visual art, and literary essays, following the human imagination wherever it entangles with trees.
It takes resources to gather and nurture these new voices, and we depend on the support of our member-subscribers to make it possible. But membership means more: by becoming a member of the Arnold Arboretum, you help to keep our collection vibrant and our research and educational mission active. Through the pages of Arnoldia, you can take part in the life of this free-to-all landscape whether you live next door or an ocean away.