An impressive oak tree grows on the quad of West Chester University, outside of Philadelphia. It is a healthy, open-grown individual measuring approximately 110 feet (33.5 meters) tall and with a trunk diameter of 64 inches (1.6 meters) at breast height. As the oldest tree on campus, it has become an important landmark for students. The tree is also a putative descendant of the first-described Bartram oak (Quercus × heterophylla) and is the largest of its kind in Pennsylvania. As such, the tree was recently recognized as a state champion, but this title remained somewhat uncertain, given the perplexing taxonomic status of the Bartram oak.
Ed Bruno, the landscape designer at West Chester University, has been working with the trees on campus for more than thirty years. Bruno was aware of an 1862 observation by the southeastern naturalist Samuel Buckley, indicating that the West Chester oak was perhaps a second-generation descendant of the original Bartram specimen—a seedling of a seedling. The original tree, however, is long gone, which meant that the West Chester oak—now approximately 170 years old—could not be directly compared to it. For Bruno, the identity of the tree became increasingly frustrating.
To provide some clarity, Bruno contacted a dozen or so oak taxonomists in 2015, requesting their opinion of the tree’s hybrid status and possible ancestry. He shared images of leaves, twigs, buds, bark, and acorns. Most recipients responded with slightly different opinions but agreed the tree was of hybrid origin. The varied answers, however, left the identity in ongoing limbo. Paul Manos, professor of biology at Duke University, agreed with the current identification of the specimen as a possible Bartram oak but suggested DNA testing would be necessary for verification. Testing would also provide an exciting opportunity to finally check hypotheses regarding the putative parents of this famous tree. The results would shed light on a two-hundred-year-old botanical mystery and further the narrative of hybridization as a frequent and important phenomenon in oaks.
History of the Bartram Oak
The original Bartram oak grew near Philadelphia, on the west bank of the Schuylkill River. In the mid-eighteenth century, the tree caught the eye of John Bartram, who was among the first practicing Linnaean-era botanists in the American colonies. Bartram traveled extensively throughout eastern North America, cataloguing and collecting native plants. While the anomalous oak, located within walking distance of Bartram’s home, resembled known oak species of the region, it possessed distinct—though somewhat ambiguous—morphological attributes, such as irregular lobing of the leaves and a range of leaf types from unlobed to lobed. This form of variation is termed heterophylly and likely prevented the specimen from being formally classified for another half century.
In 1802, French botanist François André Michaux traveled to Philadelphia where he met with John Bartram’s son, William Bartram, an accomplished botanist and naturalist in his own right, who was maintaining and growing his father’s botanical collection. During this visit, Michaux presumably observed the tree for the first time. When Michaux formally named Quercus heterophylla—coining the common name Bartram oak—in his North American silva, published in 1812, he designated the taxon as a new species rather than a hybrid. Michaux described the morphological ambiguity and suggested that although the Bartram oak resembled the laurel oak (Q. laurifolia), the leaves of that species were never lobed and the closest known population was more than one hundred miles from Philadelphia.
The newfound species status bestowed upon the Bartram oak, however, was quickly called into question, in 1814, by Pennsylvanian botanist Frederick Pursh, who had previously served as a horticultural manager at a neighboring estate, known as the Woodlands. “Of this singular species there is but one individual known, which grows on the plantation of the Messrs. Bartrams near Philadelphia,” Pursh wrote. “It probably is only a hybrid plant on that account, and cannot with propriety be considered a genuine species.” This first suggestion of a hybrid origin was followed by one hundred years of confusion and arguments between botanists as to the validity of this taxon as a distinct species, its hybrid status, and its potential parents.
Tragically, in 1842, almost two decades after the death of William Bartram, botanist Thomas Nuttall reported that the original tree had been recently cut down. Thomas Meehan, a preeminent American horticulturist, added to this report in 1853, noting that the tree had been removed because it “interfered with a view of the Schuylkill [River] from the Woodlands.” However, acorns of the tree had been collected before the removal and planted on the property and elsewhere around Pennsylvania. In subsequent years, numerous additional examples of this taxon were discovered in New Jersey, Delaware, Maryland, and New York. Though the infamous tree had been lost, this was not the end of the Bartram oak—as a lineage or a botanical mystery.
By the mid-1800s, seemingly every notable American botanist, and many from abroad, had examined either an herbarium specimen of a Bartram oak or an actual tree. But the debate continued, and in the words of botanist Arthur Hollick, looking back on this taxonomic foment in 1919, “The opinions expressed in connection with [the Bartram oak] were as diverse and heterogeneous as the trees were heterophyllous.” During this period, the Bartram oak was identified by various experts as Quercus ambigua, Q. phellos, Q. imbricaria, Q. laurifolia, Q. hemisphaerica, Q. coccinea, Q. leana, Q. tinctoria (or Q. velutina), Q. aquatica (or Q. nigra), Q. palustris, or some combination of these.
A trend did begin to emerge, however, during the latter half of the nineteenth century: the Bartram oak was clearly aligned, in some way, with the willow oak (Quercus phellos). This was based primarily on leaf morphology, with the willow oak exhibiting unlobed and entire leaf margins. Some authors believed the Bartram oak to be a lobed form or variety of the willow oak; others maintained that it was simply an anomalous willow oak specimen; and others (perhaps the majority) argued for a hybrid origin in which the willow oak was a parent. The second parent continued to be debated.
Among those that subscribed to the hybrid hypothesis were famed botanists Asa Gray and George Engelmann. Gray, in 1863, expressed the opinion that the Bartram oak was a hybrid between the willow oak (Quercus phellos) and black oak (Q. tinctoria, now Q. velutina). Engelmann, on the other hand, disagreed with this and argued, for a time, that the Bartram oak should be considered a distinct species. By 1877, however, he had clearly aligned his thinking with Gray, though he disagreed as to the second parent species: “While I was long inclined to follow Michaux in considering it as a distinct species … That it is a hybrid is most probable,” Engelmann wrote. “One of its parents is undoubtedly Phellos; for the other we must look among the lobe-leaved Black-oaks of its neighborhood, falcata, rubra or coccinea,” meaning the southern red oak, northern red oak, and scarlet oak, respectively.
At long last, in 1905, nearly one hundred years after Michaux’s recognition of the Bartram oak, a group from the New York Botanical Garden attempted to put the debate to rest, once and for all. Arthur Hollick, then the assistant curator of the garden, later reported that seventy-five acorns from a tree on Staten Island had been collected and propagated to test the hybrid hypothesis. The resulting seedlings exhibited considerable variation in leaf morphology, which could be arranged in a series according to the extent of their lobing. On one end of the spectrum were trees exhibiting the deep-lobed leaves of northern red oak (Quercus rubra), while others had narrow leaves with entire margins, similar to those of willow oak (Q. phellos). The remaining individuals were heterophyllous trees, exhibiting various combinations of red and willow oak leaf forms.
This was convincing evidence that the two parents for the Bartram oak were Quercus phellos and Q. rubra, and for a long time, this was the only hard evidence regarding the identity of the hybrid. But over one hundred years after this New York Botanical Garden study—and two hundred years after Michaux’s account was first published—we reopened the case. This time, however, we had access to DNA sequencing technologies and computational methods, allowing us to peer into the genomes of these trees and directly observe the genetic composition.
Modern Investigation
In an attempt to shed light on the identity of the West Chester tree—and to provide insights into the background of the original Bartram oak—we broadly sampled North American red oaks, including any species hypothesized to be involved in the hybrid history. We also collected material from the West Chester tree. We then used a genomic sequencing technique (restriction site-associated DNA sequencing or RADseq) to create a genetic dataset for these taxa, resulting in tens of thousands of informative DNA sites for downstream analyses.
Based on these data, evolutionary relationships were visualized with a phylogenetic tree. Much like a family tree, a phylogeny is a diagram depicting a pattern of descent and relationships between organisms. It is important to note that the behavior of hybrids in phylogenies is not straightforward and often results in one of two outcomes: the hybrid may be found as a close relative to one of the parent species, or it will be placed in an intermediate position in the tree, falling somewhere between the two parent species. Our phylogenetic analyses confirmed a close relationship of the Bartram oak with willow oak (Quercus phellos).
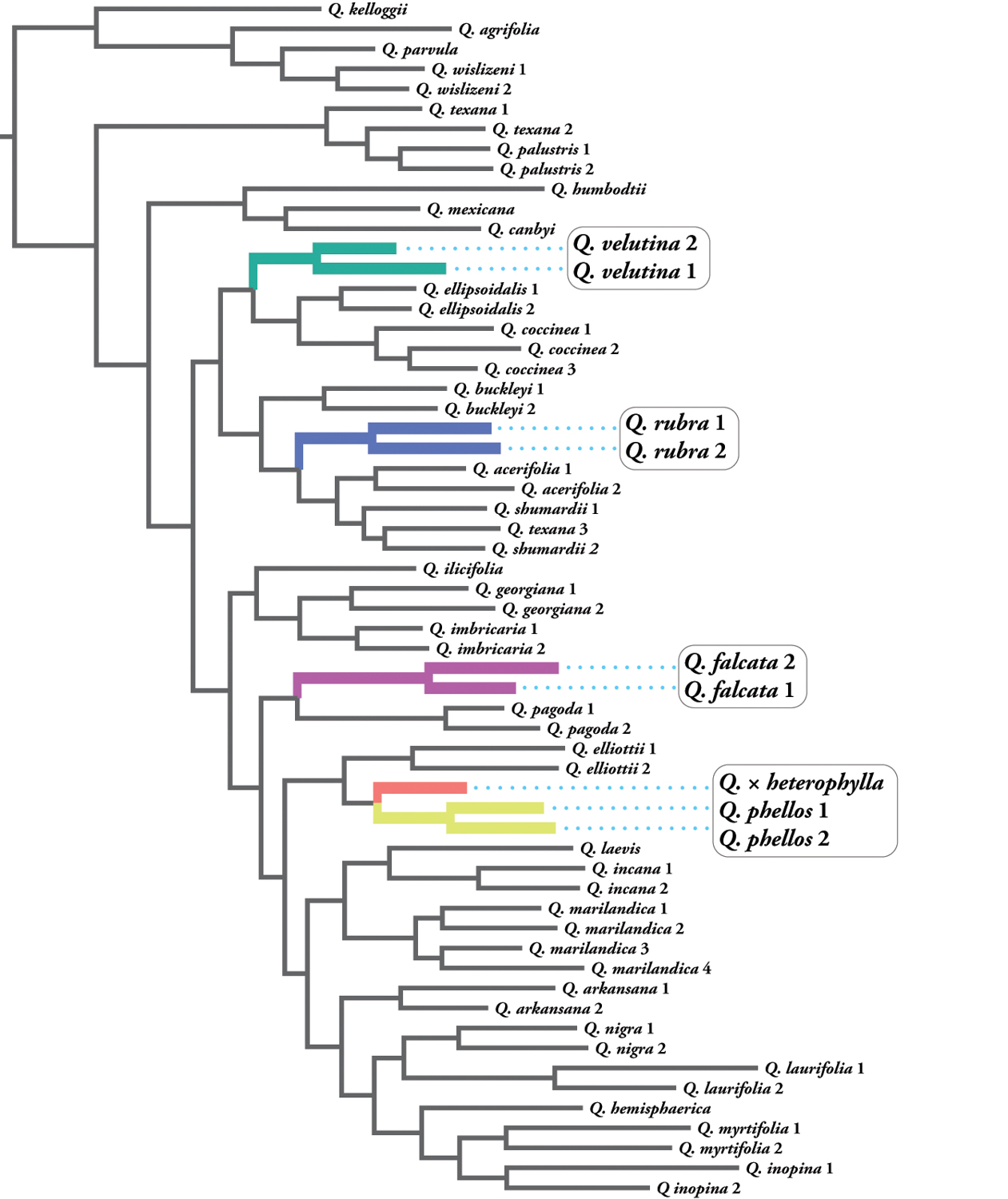


We then carried out additional DNA analyses using a clustering approach that groups individuals based on DNA similarities and differences. This technique can be used to infer the presence of admixed individuals (those whose genomes are a combination of different parent species). Based on previous assertions and our own morphological insights, we tested three plausible hypotheses regarding possible parental lineages: willow oak crosses with southern red oak (Quercus falcata), black oak (Q. velutina), and northern red oak (Q. rubra). Our clustering analyses indicated the genome of this specimen is a mosaic, suggesting a hybrid origin, with northern red oak (Q. rubra) as the probable second parent. This confirmed morphological observations of the 1905 New York Botanical Garden study, as well as our own detailed observations.
The West Chester oak shows many fruit characteristics similar to Quercus rubra. Fruit size is larger than would be expected given any of the other potential parents, measuring up to 1.2 inches (30 millimeters) in length, consistent with the large nuts of Q. rubra. The cup covering of the nut also suggests Q. rubra as a likely parent: while the cup of this taxon covers approximately one-quarter of the nut, Q. velutina and Q. falcata both possess cups that cover up to one-half of the nut. Cup scale arrangement is consistent with Q. phellos and Q. rubra, both of which have smooth and tightly appressed scales. Bud size and bud scales are also consistent with Q. rubra. Leaf pubescence is reminiscent of Q. phellos, which presents hairs early in development but becomes glabrous to sparsely pubescent later in the season. Late-season leaves of the West Chester oak are mostly glabrous, with tufts of hairs in the axils of veins on the underside, much like Q. rubra leaves, which are glabrous throughout development but with similar tufts of hair. Moreover, the bark of the Bartram oak is reminiscent of Q. rubra, with smooth patches on the trunk.
This hybrid scenario for the Bartram oak is plausible given the overlapping distributions of willow oak and northern red oak at the edges of their current ranges in eastern North America. As the West Chester tree is likely a second-generation offspring of the original Bartram oak, we propose the West Chester tree is the result of backcrossing with willow oak, a common element of the forest in the Philadelphia area.
Conclusions and Broader Implications
Many questions remain about the Bartram oak due to the inclusion of only a single individual in this study, but the interaction between the two parent species is clear. The parents share only a narrow range of ecological space, yet numerous hybrid individuals have been reported from the northern edge of the willow oak (Quercus phellos) range, distributed in disparate patches. This pattern is likely facilitated by an expanded distribution of willow oak due to land conversion during the last two hundred years, creating increased opportunities for the natural formation of Q. × heterophylla.
While we were unable to test whether all Bartram oaks are descendants of a single hybridization event, we believe it to be unlikely. Known Bartram oak specimens are often found as single individuals. In fact, a putative Bartram oak was recently identified by Paul Manos within Duke Gardens, on the campus of Duke University, after years of being noted as an anomaly by garden staff. This single eighty- to ninety-year-old tree occurs, along with both parent species, on the edge of the garden in an area that was historically forested. This suggests the Duke individual is a naturally occurring hybrid rather than an intentional planting. We posit Bartram oaks are the result of multiple independent events that have occurred repeatedly. Future studies with increased sampling will be needed to directly test this hypothesis.

Hybridization is certainly a common phenomenon in oaks; however, past concerns of oaks failing to form genetically coherent entities that merit species status have not been substantiated by genetic data. Based on recent DNA studies, we know that oak species have originated by diverging from one another in spite of gene flow. Oak hybrids are known to be fertile, and may eventually participate in forming narrow genetic bridges between species and generating new genetic combinations. This view of species as potentially open systems is based on observations made by generations of botanists. As more organisms across the tree of life are studied, the zoocentric definition of species as reproductively isolated end products of evolution is beginning to fade into history. This new paradigm redirects the question of species status to instead consider the evolutionary potential of naturally occurring Bartram oaks and the role of hybridization, in general, as oaks continue to respond to rapidly changing climates and landscapes.
The West Chester oak, in its relative isolation as a prized campus monument, is unlikely to contribute to this evolutionary continuum of gene swapping. But in natural populations, hybridization is no doubt playing a role in shaping the genetic architecture of future generations of trees. For now, and to satisfy those who need to classify and at the same time honor our rich botanical heritage, it seems fitting (and useful) to recognize all first- and later-generation hybrids of Quercus phellos and Q. rubra that show intermediate morphological qualities as Bartram oaks (Q. × heterophylla). And in the meantime, the champion West Chester tree remains a noteworthy destination for anyone with horticultural wanderlust.
Bibliography and further readings
Buckley, S. B. 1862. Note no. 2: On Quercus heterophylla, Mich. Proceedings of the Academy of Natural Sciences of Philadelphia, 14: 100–101.
Conte, J. L. Le, H. Allen, S. B. Buckley, W. M. Gabb, W. Stimpson, and E. Coues. 1861. Note on the Bartram Oak (Quercus heterophylla). In Proceedings of the Academy of Natural Sciences of Philadelphia, 13: 335–390. Philadelphia: Academy of Natural Sciences.
Engelmann, G. 1877. The oaks of the United States. Transactions of the Academy of Science of Saint Louis, 3: 539–543.
Hipp, A. L., Manos, P. S., Hahn, M., Avishai, M., Bodénès, C., Valencia-Avalos, S. 2019. Genomic landscape of the global oak phylogeny. New Phytologist. doi:10.1111/nph.16162
Hollick, A. 1919. The story of the Bartram Oak: How a little exact experimental science solved a problem of long standing. Scientific American, 121(17): 422–432.
MacDougal, D. T. 1907. Hybrids among wild plants. The Plant World, 10: 25–27.
Martindale, I. 1880. Notes on the Bartram Oak: Quercus heterophylla, Michx. Camden, NJ: S. Chew.
Meehan, T. 1853. The American handbook of ornamental trees. Philadelphia: Lippincott, Grambo, and Company.
Meehan, T. 1902. Contributions to the life-history of plants, No. XVI. Proceedings of the Academy of Natural Sciences of Philadelphia, 54: 33–36.
Michaux, F. A. 1812. Histoire des arbres forestiers de l’Amérique septentrionale, considérés principalement sous les rapports de leur usages dans les arts et de leur introduction dans le commerce. Paris: L. Haussmann.
Citation: Crowl, A., Bruno, E., Hipp, A., and Manos, P. 2020. Revisiting the mystery of the Bartram oak. Arnoldia, 77(4): 6–11
Nuttall, T. 1842. The North American sylva, or, a description of the forest trees of the United States, Canada, and Nova Scotia, not described in the work of F. Andrew Michaux. Philadelphia: J. Dobson.
Pursh, F. 1814. Flora Americae Septentrionalis; or a systematic arrangement and description of the plants of North America (Vol. 2). London: White, Cochrane, and Company.
Trelease, W. 1917. Naming American hybrid oaks. Proceedings of the American Philosophical Society, 56: 44–52.
Andrew Crowl is a postdoctoral associate at Duke University. Ed Bruno recently retired as landscape designer at West Chester University, after a thirty-year career with the school. Andrew Hipp is senior scientist in plant systematics and herbarium curator at the Morton Arboretum. Paul Manos is a professor in the Department of Biology at Duke University.