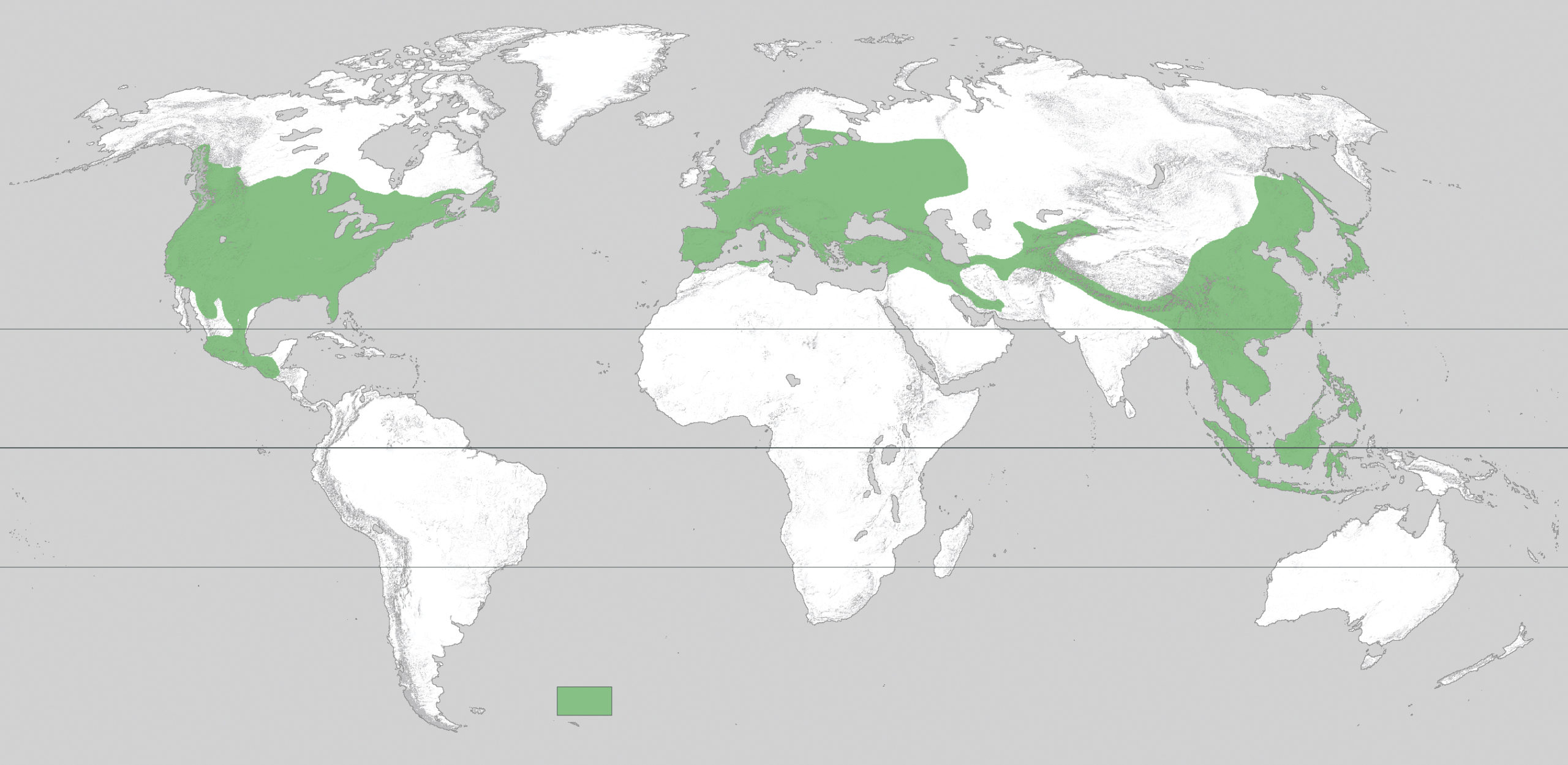
For all of human history and many millions of years before it began, the forests of the temperate Northern Hemisphere have been populated by maples. Today, the maple genus (Acer) extends its reach from Guatemala to Canada, the Mediterranean to Scandinavia, and Southeast Asia to the Amur Valley. Like oaks, willows, and birches, among many other genera, the maples as we know them today differentiated from their nearest relatives at a time when the global climate was hotter and wetter than today’s and have since survived a long period of cooling and drying, including many ice ages. Their evolution as a genus occurred through geographical radiation across the Northern Hemisphere, interspersed by extinctions and range retractions when climatic conditions became inhospitable.
Contemporary maple diversity is the result of this history and represents only a single, still snapshot from a larger, unspooling reel. The extant maples have adapted, by and large, to climatically temperate conditions: warm summers and cold winters, with occasional dry periods interspersed with regular precipitation. But contemporary, human-caused climate change is rapidly reconfiguring this climate to a warmer one with less regular and more extreme events of rain or snow, making freakish droughts, early arrivals of spring, and warm winters more common. As the climate changes, maples, like other forest species adapted to the temperate north, face an uncertain future.
In my research at the Arnold Arboretum, I make use of publicly available data, existing scholarship, and, most importantly, the Arboretum’s collection of over six hundred maple trees (which is nationally accredited by the Plant Collections Network) to predict how the genus will respond to climate change. Specifically, I ask how maple species differ in their response to dry soil conditions and to the shorter, warmer winters that will likely become typical in the Northern Hemisphere. In doing so, I treat maples as a model for other kinds of temperate trees.
The genus makes for a good model for several reasons. First, the maples are highly diverse. The genus consists of 120 to 160 species depending on the taxonomic authority, with half of these growing at the Arboretum. Second, maples have a very wide geographic distribution, unlike some temperate genera confined to only certain continents or regions. And third, as ecologically foundational, long-lived trees, maples are of interest in and of themselves, and so there is an existing body of research addressing their natural history, ecology, and evolution. Yet, in the final analysis, the story of the maples is powerful because it is typical: the genus is neither wildly more nor less vulnerable to climate change than other temperate woody genera. As such, maples can serve as a bellwether for other temperate trees: where goes the maple, so go other temperate taxa. Thus, the genus can tell us about the past, and potentially the future, of northern forests.
Paleogene Origins
Maples belong to the highly diverse angiosperms, or flowering plants, which probably had differentiated from their ancestors by the beginning of the Cretaceous period, some 145 to 66 million years ago (Coiro et al., 2019). During that period, the earliest flowering plants spread across the globe, competing with and living alongside the previously dominant woody gymnosperms (including pines, cypresses, and ginkgoes). But it was during the next geologic period, the Paleogene (66 to 23 million years ago), that maples split off from their relatives among the flowering plants and truly came into their own.
By the beginning of the Paleogene, the world’s continents were more or less in their present locations, although their climates and the degree of connectivity among them differed from conditions in the present day. Land bridges between North America and both western Europe (through Greenland) and East Asia (through Alaska) emerged periodically during cooler parts of this climatic cycle and sank back beneath the waves during warmer ones. In general, the global climate was hot and wet: 18°F (10°C) hotter on average than global temperatures during the twentieth century. This means that tropical biomes extended across much of the Earth’s land surface, with the poles experiencing temperate conditions like those we now have at the midlatitudes. Ice was absent—or very scarce—on the Earth’s surface. As a result, the growth and abundance of plants living at the North and South Poles was probably limited not by cold temperatures but by the scarcity of light (Tiffney and Manchester, 2001).
In these conditions, so different from those we experience sixty million years later, the population of trees that would give rise to the modern maples became distinct from its kin. Per fossil evidence—the appearance of recognizably maple-ish leaves and fruits—and complementary modeling based on the genetics of existing maple species, it was at this point that maples diverged from other genera in the soapberry family (Sapindaceae). This group, which also encompasses horsechestnuts and buckeyes (Aesculus) and lychee (Litchi chinensis), presently consists of over 130 genera and close to two thousand species. Of these, the maple genus is most closely related to Aesculus and to Dipteronia, the two extant species of which can be found in mainland China. Indeed, China is likely the evolutionary cradle of maples; despite some fossil evidence that maples originated in North America and spread to Asia over Pacific land bridges, the most recent molecular evidence points to an Asian origin (Li et al., 2019).
From these beginnings in China, maples radiated across the entire Northern Hemisphere while the warm, wet climate of the Paleogene was at its acme. Studies of fossil evidence marshaled by paleontologists such as Toshimasa Tanai (1983), Jack Wolfe (Wolfe and Tanai, 1987), and Harald Walther (Walther and Zastawniak, 2005) indicate that, during this time, the maples were highly diverse and cosmopolitan in their distribution. For example, the maple flora of western North America, for which the fossil record is particularly strong, currently consists of three species: bigleaf maple (Acer macrophyllum), vine maple (A. circinatum), and Douglas maple (A. glabrum). If generous, we could also include in this count the widespread box elder (A. negundo) and the western bigtooth maple (A. grandidentatum), which is often, and rightly, I would argue, treated as a subspecies of sugar maple (A. saccharum). Regardless, Wolfe and Tanai (1987) report paleontological evidence of ninety-one distinct maple species in the region; some of these may be the ancestors of the modern western maples, but the vast majority have been lost to extinction. This pattern, in which current maple biodiversity represents a small subsample of a formerly diverse flora, is perhaps best documented in western North America, but it likely holds true across the maples’ distribution. But why?
Maples on Ice
In short, maples can best be thought of as either pitiable victims or, perhaps, resilient survivors of tens of millions of years of adverse climate change. Starting roughly fifty million years ago, the Earth entered a long period of gradual and intermittent global cooling, one we would still be in if not for anthropogenic climate warming. During this time, the poles and middle latitudes became cooler and drier, giving rise to the ecosystems that we now associate with the high latitudes. Permanent ice formed in the Arctic, and glaciers periodically developed and spread south. As a result, maples were pushed toward the equator in some cases and restricted to small refugia—areas of permissive warm and wet conditions—in others. Those species that could not tolerate the increasingly cold and arid climate or migrate away from local, harsh conditions went extinct. At the same time, ice formation and climatic cooling opened up new land bridges. These included not only those among continents but also smaller regional bridges, connecting, for instance, mainland China to Japan, Taiwan, and the bulk of the Korean Peninsula. During these moments of connection, the maples’ migration in response to climate change occurred alongside the interchange of previously isolated floras.
Yet the decline of global maple diversity with climatic cooling and drying was not uniform. In general, the last fifty million years have been easier on the East Asian maple flora, which, protected by the geographic diversity and relatively stable climate of the region, now includes the native range of upwards of 80 percent of today’s maple species diversity. The maple floras of Europe and North America, on the other hand, have been much more vulnerable to climatic cooling, which has frequently led to considerable glaciation of both continents. However, it is important to note that these cold, dry periods of migration, extinction, and exchange were likely cyclic. As a result, maples, like many other temperate tree lineages, were squeezed and pushed, but then given periods of ten million years or so of relaxed, permissive climatic conditions. During these relaxed periods, populations likely rebounded, beneficial climatic adaptations spread, and species were able to expand from their refugia and southern havens to repopulate the north.
We can see evidence of this pattern if we consider the most recent glacial cycle, alternately referred to as the Pleistocene Ice Age or the Last Glacial Period, which ended eleven thousand years ago. At the height of this Ice Age, glaciers reached well into the northern United States, and much of what we now think of as forestland was probably devoid of tree cover. During this time, sugar maples and box elders migrated deep into Central America, returning north as the glaciers retreated and the climate warmed, rendering their southern refugia too hot and dry and opening up new territory in what is today the United States. As a result, relictual pockets of these maple species can still be found in cool, wet locations, such as cloud forests, in Mexico and Guatemala. This pattern of range shifts and adaptation to new conditions serves as a likely illustration of other maple species’ responses to climate change over the last fifty million years.
Classification from Evolution
Against this backdrop of global change and migration, sixty million years of evolution has given rise to our current maple flora of roughly 120 species. (I prefer to stick to a relatively low estimate of maple species diversity. Higher species counts—close to 160 in some cases—treat two maple populations separated by geography but capable of interbreeding as different species instead of subspecies.) As noted above, the majority of maples (more than one hundred species) are native to the genus’s ancestral East Asian home; nine are native to North America; and eleven are native to Europe and Western Asia. Furthermore, a handful of East Asian species are truly tropical, extending into mainland Southeast Asia and Indonesia. Maples are a staple of the Northern Hemisphere’s temperate forests, although their ecological role varies from canopy-spanning dominants (sugar maple, Acer saccharum, and red maple, A. rubrum, in the eastern United States) to specialists that are more sparsely distributed in the understory (moosewood, A. pensylvanicum) or generally riparian (silver maple, A. saccharinum, and box elder, A. negundo). Western botanists since Linnaeus have studied this considerable diversity among the maples (de Jong, 1994). Yet recent advances have finally made it possible to describe the genus in properly evolutionary terms.
For many contemporary biologists, one of the main goals of taxonomy—the classification of organisms—should be the creation of a system in which species are organized according to their evolutionary relationships. In such a phylogenetic approach, species in a given genus, for instance, are all descended from a common ancestral population and are thus more closely related to each other than to other species outside of the genus. This is almost certainly the case for Acer as it has been described since the authoritative taxonomy by German botanist Ferdinand Pax in 1885. His work, of course, was carried out shortly after Charles Darwin’s proposal of adaptive evolution and many decades before the advent of modern genetics, and so is based entirely on morphological comparisons.
Since Pax, students of the maple genus have continuously refined the organization of Acer, proposing and dismissing a variety of schemes in which the genus is organized into sections (each containing species more closely related to each other than to those in other sections) and, within sections, series. For instance, since 1933, botanists have generally agreed that the morphologically similar red and silver maple, both native to North America and unique in their flowering phenology, are members of a distinct section, Rubra (de Jong, 1994), with only a single, long-lost East Asian cousin (A. pycnanthum). More recently, such classifications have been put to the test through the application of modern genomic analyses.
Most recently, botanist and former Arnold Arboretum senior researcher Jianhua Li, presently at Hope College, has capped off two decades of research into maple systematics by publishing, with colleagues, a definitive phylogeny of the genus (2019). Their portrait of the genus’s diversity suggests the existence of sixteen sections, most of which had become evolutionarily distinct by roughly thirty-three million years ago. This point, marking the transition from the Eocene epoch (which began fifty-six million years ago) to the Oligocene epoch (which ended twenty-three million years ago), also coincided with a dramatic drop in global temperatures following a gradual cooling during the Eocene. By the time global cooling preceding our current age had really begun to accelerate, the maple genus had experienced its most profound evolutionary diversification. The emergence, over the subsequent thirty million years, of today’s maple species, was likely shaped by smaller-scale adaptations and the extinction of existing lineages, rather than by wholesale innovations within the clade.



So today, after tens of millions of years of evolution, what visible traits define a maple tree? Leaf arrangement and shape, and seed type are probably the best way to identify a member of the genus. To begin with, all of the temperate maples are deciduous and broad-leaved, with opposite leaves setting them apart from many other angiosperm taxa. Most have simple, palmately veined leaves with anywhere from three to thirteen lobes, giving rise to the “maple leaf” shape popularized by the Canadian flag and currency, which portray the leaf of a sugar maple—and, in some cases, erroneously, that of an invasive Norway maple (Acer platanoides). Yet not all maple leaves fit this rubric. A few species, such as the hornbeam maple (A. carpinifolium) bear simple leaves with pinnate venation. And two surviving lineages have developed compound leaves. These species include the North American box elder in one lineage and Arboretum classics such as the East Asian paperbark (A. griseum) and Nikko (A. maximowiczianum) maples in the other. Furthermore, all maples produce beloved paired samaras: dry, winged fruit that can “helicopter” away from their mother tree when ripe. The presence of a paired samara generally will mark a temperate tree as a maple, though other genera, including ashes (Fraxinus), produce unpaired samaras. A tree bearing opposite leaves and paired samaras, then, is very likely to be a maple.
On the other hand, flowers and bark are so diverse within the maple genus as to be unhelpful to most casual plant taxonomists. All maples produce regular, five-part (or rarely four-part) flowers, with fertilized female flowers eventually giving rise to samaras. Yet, here the similarities end. Flowers can be red, yellow, or green, and male or female (though male flowers often bear undeveloped ovaries). Maples can be dioecious—single-sexed—or monecious—having male and female flowers on the same plant. Monecious trees can produce waves of flowers over a single season, going from male, to female, to male again. Few trees, most notably box elder and the horned maple (Acer diabolicum), are fully dioecious, meaning they consistently present as either male or female.



Maple bark presents another lesson in the genus’s surprising diversity. The scaly motley of green and brown lining the trunk of European sycamore maple (Acer pseudoplatanus) makes these trees easy to spot, although in some cases, misleadingly similar to true sycamores (Platanus). The bright orange, peeling bark of the paperbark maple (A. griseum) is one of its outstanding merits as a horticultural tree. But it is the species of the section Macrantha, the snakebark maples, whose green, smooth-to-furrowed bark is, to me, most unusual and appealing. These species are restricted in their distribution to East Asia except for the North American moosewood maple (A. pensylvanicum).
Hot and Cold
Despite long-standing celebration of maples’ morphological diversity, ecologists still lack a clear understanding of physiological diversity in the genus. I am interested in this question out of the need to forecast how particular species of maples, as well as other temperate trees, will respond to climate change. Recent studies have already documented some climate-related shifts in maple distributions. For instance, in North America, red maples seem to be increasing in abundance, while sugar maples are in decline (Fei and Steiner, 2007; Oswald et al., 2018). But how can these patterns be generalized across the remainder of the genus? Will those species that already live in warmer and drier climates be favored by our warming and increasingly drought-prone anthropogenic climate? Or are species from cooler, wetter habitats secretly concealing a capacity to put up with a wider range of conditions than indicated by their current distribution? In my ongoing study of the climate-change vulnerability of the maples, I seek to answer these questions.
Most temperate forests will experience hotter conditions and a greater risk of drought as our climate changes. What will this mean for the maples? In comparisons of diverse woody plants from across the globe, a few physiological traits have emerged as excellent predictors of how good a given species is at thriving in hot, dry conditions. One of these is turgor loss point, the water potential at which a leaf from a tree or shrub loses turgor, or wilts (Bartlett et al., 2012). To date, I have measured turgor loss point for seventeen species of maple coming from diverse sections of the genus and from all over the world.
One pattern emerging from these data is that European and West Asian maples have the lowest (most drought tolerant) turgor loss points, followed by North American and then East Asian species. It appears that species living in the genus’s original homeland are among the most intolerant maples of hot, dry conditions. This could be due to differences in the climatic histories of the maples’ East Asian, North American, and European ranges. In Europe, for instance, cycles of glaciation and warming have likely pushed to extinction any species that could not survive dry conditions; East Asia likely contained more refugia, allowing these species, including many maples, to survive to the present (Tiffney and Manchester, 2001).


Yet the perils of hot and dry conditions are not the only challenge that climate change will pose to the temperate maples. Paradoxically, some temperate trees may be at greater risk of springtime freezing damage in a warming climate. The increasing likelihood of false spring events, in which warm, short winters allow plants to begin growing again earlier in the year, may lead some trees to lose cold hardiness and sustain critical damage from sudden drops in late winter temperatures. To understand whether maples will be sensitive to false springs, I partnered with my colleague and fellow postdoctoral Putnam Fellow Al Kovaleski, an expert in measuring cold hardiness in woody plants. In these tests, we generally find that maples can withstand much colder temperatures than they are likely to experience in their native habitats—or in Boston!
Many of our test species, even when actively growing, flowering, or putting out new leaves for the spring, can withstand freezing to 14°F (-10°C) or below. It seems unlikely that false springs concomitant with a warming climate will expose trees to these temperatures at the right time of the year and for the periods of time necessary to cause considerable damage. However, in our analysis of cold hardiness across species, we found a continental pattern that echoed my work on tolerance of drought. East Asian species were, once again, least tolerant of cold conditions. But, in contrast to intercontinental differences in turgor loss point, North American species were generally the most cold hardy, with European species intermediate and East Asian species most vulnerable. This is likely due to differences in the way that each continent experiences the onset of spring. Recent work by a group of ecologists led by Constantin Zohner (2020) has established that North American forests have historically been much more likely to experience freezing temperatures in late spring. European and Asian floras have responded to generally more permissive springs by developing an opportunistic strategy, taking more risks and initiating growth earlier in the spring. As a result, European trees are thought to be in the most danger of damage from false springs as the climate continues to warm.
Back to the Future
But what do these findings mean for the climate-change resilience of maples and, by extension, other temperate woody species? For me, the best way of thinking about the future is to once more turn to the genus’s past. For instance, though it’s impossible to measure the physiological diversity of the historic European and North American maple floras, we might imagine that, under warmer and wetter conditions, in which environmental pressures were laxer, each region was home to maples adapted to a variety of environments. But paleontological evidence, climate modeling, and the present-day existence of only drought-tolerant European maples and relatively cold-tolerant American species suggest a compelling story.
Successive cycles of cold, dry, and glaciated ice ages during the Oligocene, interspersed with warming, may have weeded out those species sensitive to these environmental stressors. This mechanism has been suggested to explain the relative lack of diversity in temperate eastern North American and Eurasian forests relative to those in East Asia and western North America (Qian and Ricklefs, 2000; Svenning, 2003). And they certainly explain patterns of physiological and species diversity for the extant maples outside of East Asia. Perhaps the few remaining species in North America and Europe are those that have managed to survive, adapt, and migrate in response to glaciations and accompanying cold and dry conditions. Pushed into Meso- and Central America and into Northern Africa, they have subsequently returned to higher latitudes, becoming locally abundant in the case of widespread species such as sugar, red, Norway, and sycamore maples, among others. Differences in the geography and climate of each continent have only reinforced these adaptive patterns: North America, for instance, has more extreme and variable early spring temperatures, producing a more cold-hardy flora (Zohner et al., 2020). But what will happen next, as our climate warms and patterns of precipitation become more erratic?
We could be facing a future reminiscent of our past, but with a twist. Paleoecologist Kevin Burke and colleagues, in 2018, offer a particularly compelling illustration of how global change might affect Earth’s temperate forests. In this modeling exercise, the authors compare likely scenarios of future climate change to what we know about the Earth’s historical climate based on climatological reconstructions. Disturbingly, they predict that, under a business-as-usual scenario, in which humans do nothing to curb climate change, the Earth very well may, by 2200, have a climate akin to that of the Eocene period (roughly fifty million years ago in their analysis). This means that, in just seven maple generations, we may skip over fifty million years of changing climate, reverting to conditions similar to those before the diversification of the maple clade in the late Eocene and early Oligocene. Whether our current palette of maple species can tolerate these conditions is unclear.
Our warming climate could open up some habitat, at least initially, for cold-tolerant and northerly distributed maples (like sugar maple and Norway maple) to extend further into areas that are presently too cold for trees to inhabit. And maybe the highly drought-tolerant species of Eurasia will be able to capitalize on drying and warming conditions to displace more-mesic species. But there is also a likelihood that anthropogenic climate change will create what climatologists call no-analog conditions, a climate unlike one that our existing flora and fauna have ever experienced, much less adapted to. In this case, humans will truly face something new under the sun, as will our biotic companions. In the immediate future, the story of the maples suggests the critical need to begin the conservation of woody plants we know to be heat- or drought-intolerant. These conservation efforts include the ex situ migration of species out of their current native ranges. We also should begin investing in forestry that focuses on those species that, by virtue of their natural history, have proven themselves capable of withstanding long periods with limited access to water.
These suggestions, based on the climate-change vulnerability of the maples, are meant to apply to a variety of temperate woody taxa, including oaks, willows, and birches. Each of these genera is the result of a complex set of journeys across multiple continents and survival over many millions of years of global change. That such adaptation is possible should at least give us hope for the work ahead of us to keep the planet livable, both for our own progeny and for whatever comes next in the story of the Northern Hemisphere’s forests.
References
Bartlett, M. K., Scoffoni, C., and Sack, L. 2012. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: A global meta-analysis. Ecology Letters, 15: 393–405.
Coiro, M., Doyle, J. A., and Hilton, J. 2019. How deep is the conflict between molecular and fossil evidence on the age of angiosperms? New Phytologist, 223: 83–99.
Fei, S., and Steiner, K. C. 2007. Evidence for increasing red maple abundance in the eastern United States. Forest Science, 53: 473–477.
Gibbs, D., and Yousheng, C. 2009. The red list of maples. Richmond: Botanic Gardens Conservation International.
de Jong, P. C. 1994. Taxonomy and reproductive biology of maples. In D. M. van Gelderen, P. C. de Jong, and H. J. Oterdoom (Eds.), Maples of the world (pp. 69–99). Portland, OR: Timber Press.
Li, J., Stukel, M., Bussies, P., Skinner, K., Lemmon, A. R., Lemmon, E. M., Brown, K., Bekmetjev, A., and Swenson, N. G. 2019. Maple phylogeny and biogeography inferred from phylogenomic data. Journal of Systematics and Evolution, 57: 594–606.
Oswald, E. M., Pontius, J., Rayback, S. A., Schaberg, P. G., Wilmot, S. H., and Dupigny-Giroux, L. A. 2018. The complex relationship between climate and sugar maple health: Climate change implications in Vermont for a key northern hardwood species. Forest Ecology and Management, 422: 303–312.
Qian, H., and Ricklefs, R. E. 2000. Large-scale processes and the Asian bias in species diversity of temperate plants. Nature, 407: 180–182.
Svenning, J.-C. 2003. Deterministic Plio-Pleistocene extinctions in the European cool-temperate tree flora. Ecology Letters, 6: 646–653.
Tanai, T. 1983. Revisions of Tertiary Acer from East Asia. Journal of the Faculty of Sciences, Hokkaido University, 20: 291–390.
Tiffney, B. H., and Manchester, S. R. 2001. The use of geological and paleontological evidence in evaluating plant phylogeographic hypotheses in the northern hemisphere tertiary. International Journal of Plant Sciences, 162: S3–S17.
Walther, H., and Zastawniak, E. 2005. Sapindaceae (Aceroideae) from the late Miocene flora of Sos´nica near Wrocław – A revision of Göppert’s original materials and a study of more recent collections. Acta Palaeobotanica, 45: 85–106.
Citation: Grossman, J. J. 2020. Model maples. Arnoldia, 78(1): 6–15
Wolfe, J. A., and Tanai, T. 1987. Systematics, phylogeny, and distribution of Acer (maples) in the Cenozoic of Western North America. Journal of the Faculty of Sciences, Hokkaido University, 22: 1–246.
Zohner, C. M., Mo, L., Renner, S. S., Svenning, J.-C., Vitasse, Y., Benito, B. M., Ordonez, A., Baumgarten, F., Bastin, J. F., Sebald, V., Reich, P. B., Liang, J., Nabuurs, G. J., De-Migueln, S., Alberti, G., Antón-Fernández, C., Balazy, R., Brändli, U. B., Chen, H. Y. H., Chisholm, C., Cienciala, E., Dayanandan, S., Fayle, T. M., Frizzera, L., Gianelle, D., Jagodzinski, A. M., Jaroszewicz, B., Jucker, T., Kepfer-Rojas, S., Khan, M. L., Kim, H. S., Korjus, H., Johannsen, V. K., Laarmann, D., Langn, M., Zawila-Niedzwiecki, T., Niklaus, P. A., Paquette, A., Pretzsch, H., Saikia, P., Schall, P., Seben, V., Svoboda, M., Tikhonova, E., Viana, H., Zhang, C., Zhao, X., and Crowther, T. W. 2020. Late-spring frost risk between 1959 and 2017 decreased in North America but increased in Europe and Asia. Proceedings of the National Academy of Sciences of the United States of America, 117: 1–9.
Jake J. Grossman is a visiting assistant professor of ecology at Swarthmore College and a research associate at the Arnold Arboretum. From 2018 to 2020, he was a Putnam Fellow at the Arboretum.
