The largest [Japanese plum yews] I saw grow in the rich forests at the foot of Higashi-Kirishima … I saw many trees from 8 to 10 m tall with … wide-spreading branches forming broad rounded crowns. Such trees, with their dark-green leaves pale or glaucescent on the under side, are very beautiful…. E. H. Wilson, The Conifers and Taxads of Japan, 19161
Wilson was describing the surprisingly large specimens of Cephalotaxus harringtonia he had seen —growing in Kyushu, Japan. Although it was Wilson who introduced the plant into cultivation in the United States, he was not the first Western plant explorer to collect it and extoll its beauties. Long before, around 1829, the prominent plant collector and principal author of Flora Japonica, Philipp Franz von Siebold, had sent Cephalotaxus to Europe where it was received with interest and appreciation.2 Siebold grew five different Cephalotaxus in his own garden in Japan, along with many other plants he had discovered, cultivating them for their beauty and for evaluation as garden plants.
Today, although plum yews are widely considered some of the most beautiful and useful of evergreen conifers, their potential as ornamental and medicinal plants has yet to be fully explored and utilized. The endangered status of Cephalotaxus in the wild—particularly in China, its “distribution center and refuge,”3 where it is vulnerable to the increasing demands of an exploding human population—lends a sense of urgency to efforts to learn more about this fascinating genus. At the Arnold Arboretum, we are working to help conserve Cephalotaxus while continuing to study and propagate the genus for use in cultivation.
Cephalotaxus
The modern natural range of Cephalotaxus has diminished considerably from that of its early antecedents. Now the genus is restricted to southern and eastern Asia—Japan, Korea, south, central, and eastern China, Hainan, Taiwan, India, Burma, Laos, and parts of Vietnam.4 Cephalotaxus was also found in Europe and northwestern North America in the Miocene and Pliocene eras; moreover, during the Jurassic era its antecedents extended into what is now Greenland.5

Because Japanese plum yew has been in cultivation in Europe and the United States for close to a century, many modern horticulturists are familiar with the Japanese species Cephalotaxus harringtonia, named in honor of the Earl of Harrington, one of the first to grow the plant in a European garden. Far fewer are aware of other equally beautiful members of the genus that were not found by Western explorers until the turn of the century. Six to twelve species and botanical varieties, depending on the taxonomist consulted, comprise an elegant genus with an inelegant name.
While today Cephalotaxus is most often considered the single genus of the coniferous Cephalotaxaceae, it was earlier included in the Taxaceae with taxads like Torreya, Taxus, and Pseudotaxus.6 Distinctive aspects of the embryogeny and development of Cephalotaxus set it apart from this group, however, in spite of shared adult morphological characteristics like fleshy seed coats, two-ranked needles of similar shape, and low shrub to small tree habits.7 A few modern authors include Amentotaxus in the Cephalotaxaceae, resulting in occasional references in the literature to two genera in the Cephalotaxaceae.8
Plum yew’s botanical name is apt. “Cephalotaxus” means “head-yew,” from the Greek “kephale” for head and the botanical name “taxus” for the yew genus. “Head-yew” refers to the flowering structures that are borne in tight clusters or “heads” and to its needles, which resemble those of yew. Another, more appealing common name, plum yew, refers to the plum-like shape and color of the ripened fleshy “cone.”
Cephalotaxus is most often found growing as shrubs or small trees in soils rich in humus in moist subtropical or warm-temperate forests, generally as understory plants in at least light shade. They are primarily low- to mid-altitude plants, but a few variant types are found at higher elevations and on chalky gravel cliffs. The entire range of the genus, however, extends from tropical to cool temperate climates, and cold hardiness of cultivated taxa corresponds to provenance.
While the foliage of plum yews generally resembles that of true yews, the reproductive strobili are quite distinct. Most of us are familiar with the bright red (or occasionally yellow), fleshy, nonpoisonous “aril” that incompletely surrounds the yew’s very poisonous, small, rounded seed. Fewer are likely to be familiar with the seed of plum yew, which is significantly larger than that of yew, being about the size and shape of an olive or very small plum (0.75 to 1.25 inches long and 0.25 to 0.75 inches wide) and completely enclosed by a thin, hard shell and an outer fleshy coat. As the seed ripens, the fleshy coat changes color, maturing from an attractive, glaucous blue-green, through a warm cinnamon-red (hence “plum yew”), and finally to a dull tan or purple-brown before abscission of the entire “cone” and/or degradation of the fleshy tissue.
Male and female plum yew strobili are borne on separate plants. Male strobili develop in flattened heads of numerous small clusters of anthers, about 0.25 inches in diameter, regularly arranged in the axils of the needles along thelength of the branchlets. Female strobili develop as clusters of six to twelve ovules in pairs held on an odd-looking, oval, initially mauve-colored head (or “cone”), that expands from about 0.5 inches in length at first visibility to the mature length of 0.5-1.25 inches (depending on the species). Usually only one seed matures per head.9 Three to five female heads are borne on stalks at or near the end of the current or previous year’s branchlets.10 Female cones are wind pollinated.
Seeds of Cephalotaxus have a relatively long period of development. Depending on species and region, pollen cones require nine to eleven months to mature (from initiation to pollen dispersal), while female cones can take as long as twenty-one months, generally maturing at the end of the second growing season after initiation.11
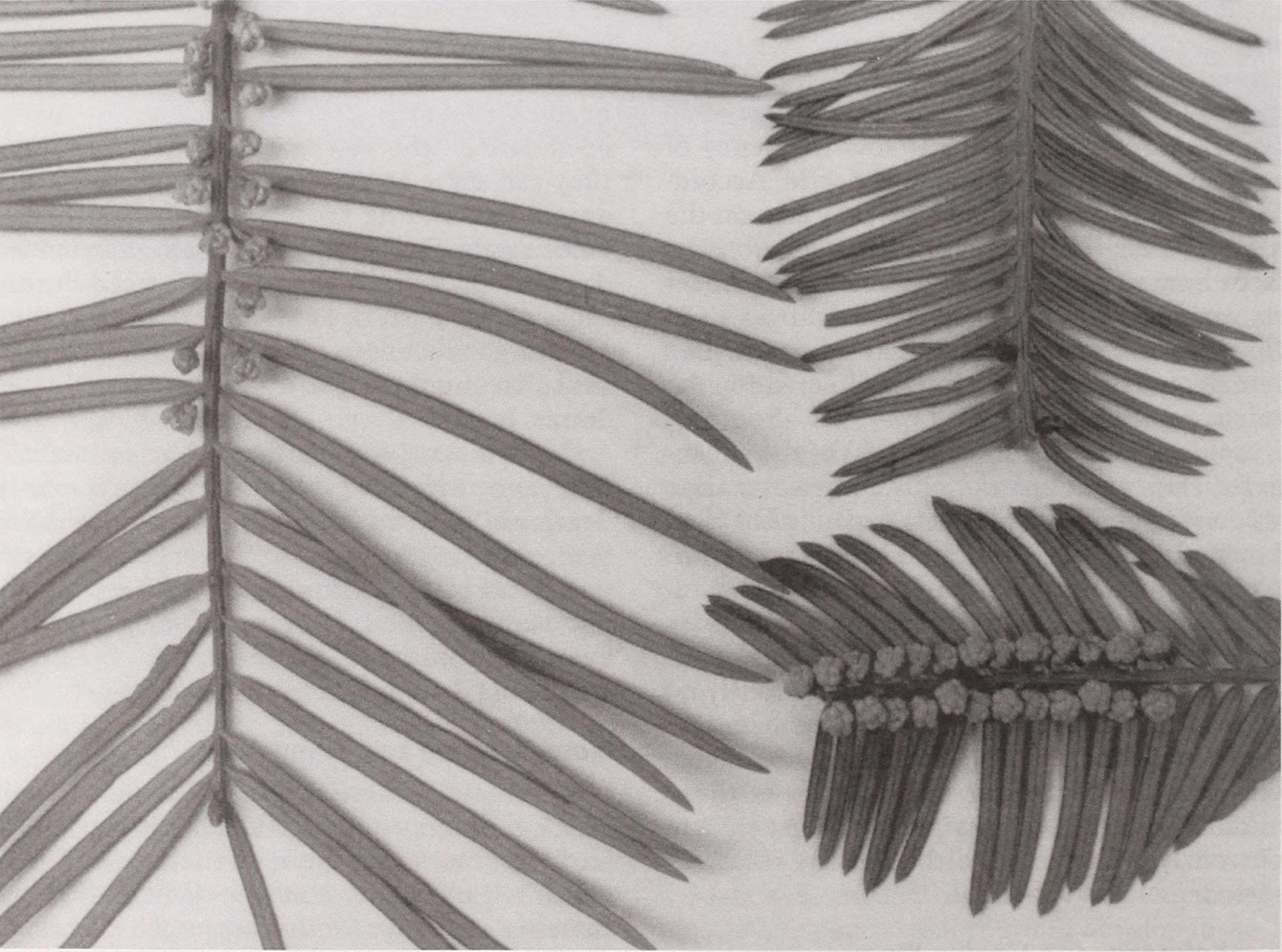
Cephalotaxus is now rare and endangered in significant areas of its range.12 Its lengthy seed maturation period, combined with a dioecious reproductive habit and an often sparse natural distribution throughout much of its range, may contribute to the seemingly low frequency of regeneration for the genus in the wild. According to Huang, animals and birds may also eat the seed.13There is also pressure on Cephalotaxus from human activity. It is harvested for timber in various parts of its range as well as used for firewood and for medicinal purposes. The female cones are sometimes collected for the oil expressed from the seed. 14
Ironically, increasing awareness of the endangered status of Cephalotaxus comes at a time when its potential value has expanded beyond horticultural uses to include anticancer compounds found in its seed and vegetative tissues. Experimental work with the ester alkaloids cephalotaxme, harringtonine, and allied chemicals has shown promise, although apparently no widespread therapeutic applications have yet been introduced.15 Sadly, two of the three species that are especially rich sources of these alkaloids, C. hainanensis and C. oliveri, are currently endangered, although the third, C. fortunei, is less vulnerable.16
Cephalotaxus as a Garden Plant
The various taxa of Cephalotaxus are of interest and value not only as endangered sources of useful materials, but as exquisitely beautiful evergreens for a variety of modern landscapes, combining graceful habits and foliage with the tough stress resistance and ease of maintenance required by modern gardeners and landscape contractors.
Cephalotaxus are slow-growing conifers with dark olive to black-green foliage. Because their habits range from upright and shrubby to low and informally mounding, they can serve as hedges, masses, groundcovers, specimens, and foundation or container plants. They thrive in a variety of soils, including extremely dense clays. They are not only tolerant of shade but—with only one exception—perform well even in heavy shade, an unusual trait for a needled evergreen. Indeed, most Cephalotaxus produce the best foliage when given at least some shade, although some maintain excellent foliage color in either full sun or shade.
Plum yews are extraordinarily heat tolerant in humid climates, another unusual trait for a needled evergreen. For this reason, they have been called “the yew of the south,” although they can serve as excellent landscape plants in an area extending far beyond the Southeast. Once established, they are tolerant of extended dry periods such as those experienced during most of our eastern summers. However, they are not good choices for hot, dry climates like those in much of the southwestern United States.
Cephalotaxus are relatively deer resistant (I have come to believe that no evergreen is totally deerproof). Deer feeding on plum yews have been reported in areas with very heavy deer populations (for example, central New Jersey and Pennsylvania). Even in these cases, however, with only one exception, deer turned to Cephalotaxus foliage only as a last resort.
Nomenclature and Taxonomy
Unfortunately, there is no current monograph on Cephalotaxus available. This is especially troublesome since the nomenclature of this genus is particularly confusing and is likely to remain a challenge for the foreseeable future; to the best of my knowledge, no taxonomic monograph of the entire genus is currently underway. Hence, one must simply dive in and make a first attempt at creating some order out of the chaos.
Key characters listed in the literature have rarely been useful to me when dealing with plum yews. I have observed that widely cited key characters such as stomatal band whiteness, length and shape of needle, and bark color can vary with age of the plant and the microenvironment in which it is grown.17 Full sun, cool temperatures, and leaf maturity, for example, appear to promote whiteness of the stomatal bands on plants of the four species now grown in North America. In another case, an oft-cited, characteristic V-shaped trough formed by the angle at which needles are held—which has been used to separate what is now called C. harringtonia var. drupaceae from the rest of the species18—can frequently be seen on plants of various species.
What this translates to on a practical basis is that confirmed provenances and commercial sources are critical when working both with species and with cultivars. In the case of species, identifying individual plants is especially challenging because the key characters are mostly morphological intergrades. Therefore, knowledge of geographic origin is important, and even when armed with such knowledge only the morphological extremes of the genus (e.g., C. fortunei with very long needles versus the shorter-needled C. harringtonia) can be reliably and consistently separated from each other ex situ. Judging from what I have observed on diverse live plants and herbarium specimens, a pragmatic taxonomist might argue for including much of the genus in a single species, at least for plants found on the Asian mainland. The following discussion of forms offers a brief introduction to the diversity of plum yews.
Cephalotaxus fortunei Hooker (Fortune’s plum yew, San-chien-shan, Lo-han-shu, three-pointed fir)
Fortune’s plum yew is native to China, where in addition to wild populations, it is found planted near shrines and temples. This species has a widespread range in central and eastern China south of the Yellow River and has been collected in Shui-sa-pa (the “Water Fir Grove” near the border of Hubei and Sichuan provinces) as part of the Metasequoia flora.19 It was introduced to both Europe (around 1849) and the United States (around 1858) by Robert Fortune, who collected it in China.20
The needles of Cephalotaxus fortunei are the longest of the genus, varying from two to over four inches; the most dramatically long-needled plants are the most elegant. Needle diameter ranges from extremely slender (1/16th inch) to nearly as wide as that of other species (1/6th inch), with color of the stomatal bands on the undersides of the needles varying from bright white to green. Bark is reddish-brown to dark brown and peels in plates as plants age. Mature female cones are longer (one-and-a-half to two inches) and often narrower than those of other species.

Cephalotaxus fortunei is a multistemmed shrub or small tree with an open, loosely rounded habit and slightly pendant branchlets. Height and spread will vary with provenance of seedlings and the climate in which the plants are grown. In China, depending on locale, C. fortunei is found as a shrub or as a small to medium-sized, multitrunked tree reaching heights in the range of thirty feet.
In Europe and North America, warmer regions give faster, more upright growth, while cooler temperatures lead to shrubbier, slower-growing plants. All of the C. fortunei selections I have seen do best in shade, which results in a more open habit than is found in sunny situations; in North America, full sun usually causes at least some winter burn on the foliage. They prefer moist, loamy soil, but will also stand up to heavy clays if grown in light shade. They are reliably cold hardy through zone 7, and in sheltered, shaded sites, into the warmer parts of zone 6.
Cephalotaxus fortunei var. alpina Li is a low form found in the mountainous forests of northwestern Yunnan and western Sichuan. C. fortunei ’Grandis’ is an especially long-needled female form, originally from Hillier Nurseries. C. fortunei ’Lion’s Plume’ is yet another long-needled cultivar, originally received in the 1950s at the Willowwood Arboretum in New Jersey but no longer in the collections there. C. fortunei ’Prostrate Spreader’ (’Prostrata’) is a long-needled, low, mounding form, also from Hillier Nurseries, with lovely dark-green foliage; several other prostrate selections available in the United States may or may not be clones of the Hillier Nurseries plant.
Cephalotaxus griffithii Hooker (Griffith’s plum yew)
Griffith’s plum yew is one of the species found in India, specifically in the Mishmi Hills of Assam (at about 6000 feet) where it is a small tree fifteen to thirty feet in height. It is also found in western Sichuan, China. Needles are two to three inches long by 1/8th inch wide. Herbarium specimens of this species appear similar to those of the geographically overlapping species C. mannii, C. oliveri, and C. sinensis.21 In the past, C. griffithii was cultivated at Kew, which received specimens from the Calcutta Botanical Garden sometime before 1890,22 but Kew’s inventory does not currently list this species. I have not seen it in cultivation anywhere in the United States. Cold hardiness of this species outside of Asia is unknown.
Cephalotaxus hainanensis Li (Hainan plum yew)
Hainan plum yew is a tropical species found on the island of Hainan, China. Some authors include this taxon as part of C. mannii, which appears to be its closest relative. On Hainan, it can grow to tree size, reaching fifty to seventy feet in height. Needles are long and slender (two or three inches by 1/8th inch); most herbarium specimens appear nearly identical to those of C. mannii except for a greater variability in needle length. Because of timbering and bark stripping, Hainan plum yew is seriously threatened in its natural range; it is also one of the species rich in anticarcinogenic alkaloids. It is not in cultivation in this country but is likely to be cold hardy only into zone 9.
Cephalotaxus harringtonia (Forbes) Koch (Harrington’s plum yew, Japanese plum yew, Inugaya)
This was the first plum yew to be collected by Westerners and has been longest in Western cultivation. It is widespread in Japan from Kyushu north to Hokkaido and is also found in areas of northeastern China and Korea. In the warmer parts of its range it is usually seen as a small tree; in colder areas it most often appears as a rounded shrub of low to medium height. It is this latter habit that most frequently develops in cultivation in Europe and North America. Its needles are relatively short and often wider than those of mainland taxa (one-to-two inches long and 1/6th inch wide), and its fruits are rounded-ovoid. The numerous cultivars have a variety of shapes, sizes, and foliage variegations.



Siebold first sent this plant to the Leiden Botanical Garden in 1829 as Cephalotaxus drupaceae. Most modern authors separate C. harringtonia var. drupaceae from typical C. harringtonia. The primary difference appears to lie in the arrangement of the needles on the stem. In the literature, both historical and modern, the foliage of C. harringtonia var. drupaceae is repeatedly described as distinctive in its upright V-formation, but I have seen this characteristic on any number of Cephalotaxus species and cultivars in diverse sites. In North America (and in a brief survey of southern England), the V-shaped character appears to be more closely related to cultural conditions and to the stage of development of the needles and plants than to any consistent taxon-specific morphological trait. This V-shaped characteristic becomes especially pronounced on the flowering branches of many male Cephalotaxus, regardless of species or variety, as pollen-bearing strobili expand in the needle axils and appear to promote “lifting” of the two-ranked needles into a V-shaped trough. The degree of “V” also increases somewhat throughout the season on all plants of various species as leaves mature and in response to dry periods. I have had no success separating what is called C. harringtonia var. drupaceae from C. harringtonia in North America by relying on these morphological characteristics. Cold hardiness and landscape performance of C. harringtonia vary with cultivar and botanical variety as noted below.
Cephalotaxus harringtonia var. nana Nakai (Hai-inugaya) is the variety found growing on seaside cliffs and mountamous areas of Hokkaido and eastern Honshu.23 Its needles are shorter and more slender than those of C. harringtonia, and the plants themselves are shorter, with a more upright, suckering habit. Its fruits are also smaller. In the wild, C. harringtonia var. nana spreads by layering; it does the same in cultivation, albeit slowly. Overall it is more compact and more finely textured than the species and retains this habit in cultivation. Plants grown from collections made by Spongberg and Weaver have been cold hardy in zone 6 at the Arnold Arboretum where foliage color remains attractive throughout the winter in the shade but bronzes heavily in full sun.24 C. harringtonia var. nana has a distinctively demure character in the landscape, and it would make a lovely small evergreen for shaded sites.
Cephalotaxus harringtonia ’Duke Gardens’ is a broadly rounded, dense shrub reaching about six feet by six feet in about ten years, depending on where it is grown. It was selected at Sarah P. Duke Gardens at Duke University in North Carolina. It makes a beautiful mass in sun or shade in zones 7 to 9 and thrives in soils from sandy loams to clays.
Cephalotaxus harringtonia ’Fastigiata’ is a distinctive upright cultivar with dark green needles whorled around the stem m a bottlebrush manner. ’Fastigiata’ grows even more slowly than the average Cephalotaxus, retaining its broad columnar habit for the first ten to twelve years before beginning to spread into a multibranched, upright mound. It does best in part shade; full shade causes it to open up and become untidy, while full sun can result in winter burn in severe years. ’Fastigiata’ is reliably cold hardy through zone 6 and much of zone 5, especially in walled gardens and other semiprotected areas, but it will suffer from snow and ice damage in severe winters. C. harringtonia ’Fastigiata Aurea’ is nearly identical to ’Fastigiata’ except that its needle margins are gold.
Cephalotaxus harringtonia ’Fritz Huber’ is a low-spreading cultivar with stiffer branches and a stiffer habit than other low-mounding types. Its needles are a brighter, more emerald green than other selections. C. harringtonia ’Gnome’ is a dwarf, rounded mound growing to two feet in height, with light green foliage and shorter, stiffer needles than the species. It is a striking, impish little plant from Hillier Nurseries. C. harringtonia ’Korean Gold’ (’Ogon’, ’Ogon Chosen Maki’)25 is identical to ’Fastigiata’ except that new growth emerges bright yellow-gold in spring and fades to green in summer. Also, its growth is slower than that of ’Fastigiata’. ’Korean Gold’ is very effective in the spring garden.
The name Cephalotaxus harringtonia ’Prostrata’ is generally applied in this country to any and all selections with a low-spreading, low-mounding habit—plants often have somewhat pendant branchlets as well. However, it should, at this time, be used only for the Hillier Nurseries selection.26 (See “A Plethora of ’Prostrata’s” on page 35.) The true Hillier Nurseries cultivar ’Prostrata’ is especially tolerant of full sun and shows no foliar burn in the northeastern United States, where other forms do burn. With its particularly pleasing, informally irregular, cloudlike silhouette, it is one of the most beautiful and useful selections of plum yew available to gardeners. Its quality was recently recognized by the Pennsylvania Horticultural Society with a Gold Medal Award. There is a reliably named, exceptionally handsome old planting of C. harringtonia ’Prostrata’ at the Brooklyn Botanic Garden.
Cephalotaxus koreana Nakai (Korean plum yew)
Korean plum yew is found at low to middle elevations in Korea, northern and central Japan, and northeastern China. It is an upright, slowgrowing shrub with broad, relatively coarse, black-green needles (about two inches by 1/6th inch). Plants will reach eight to ten feet in as many years, with a narrow spread. Its dense branching and foliage cover make this species one of the most effective for massing. It retains its remarkably beautiful black-green foliage throughout the entire year, even in an exposed winter site at the Arnold Arboretum (zone 6) where other species have bronzed heavily. Cold hardiness will vary with provenance, but C. koreana is hardy at least through zone 6 and likely into zone 5. Further collections from the coldest parts of its range would be desirable.


Cephalotaxus lanceolata Feng
C. lanceolata is known from only a few places in northwestern Yunnan Province. It closely resembles C. fortunei; indeed, the majority of herbarium specimens are practically indistinguishable from those of C. fortunei. Its needles are long and slender (about three inches by I/8th inch) and often with needle edges that are distinctly parallel up to a sharply acute apex (as opposed to tapering more gradually to an acuminate apex). Chinese authors distinguished this species from C. fortunei on the basis of its wider, thinner needles with sharper apices (hence its name, lanceolata).27
Cephalotaxus mannii Hooker (Mann plum yew)
This species grows into a tree of about seventy feet in height. It is the southernmost taxon and can be found at low to middle elevations on moist, shaded slopes and gullies in woodlands in southern China, northeastern Burma, India, Laos, and Vietnam (and Hainan if one includes C. hainanensis within C. mannii). C. mannii is sparsely distributed and seriously endangered by harvesting for timber and for medicinal purposes. Its foliage as seen in illustrations and on herbarium specimens is slender, gracefully tapering, and variable in length;28 even in a dried state it is strikingly beautiful. C. mannii is not in cultivation in the United States and is not likely to be cold hardy north of zone 9. It is exciting to learn that the Royal Botanic Garden at Edinburgh has recently acquired cuttings of this species for propagation.
Cephalotaxus oliveri Masters (Oliver plum yew)
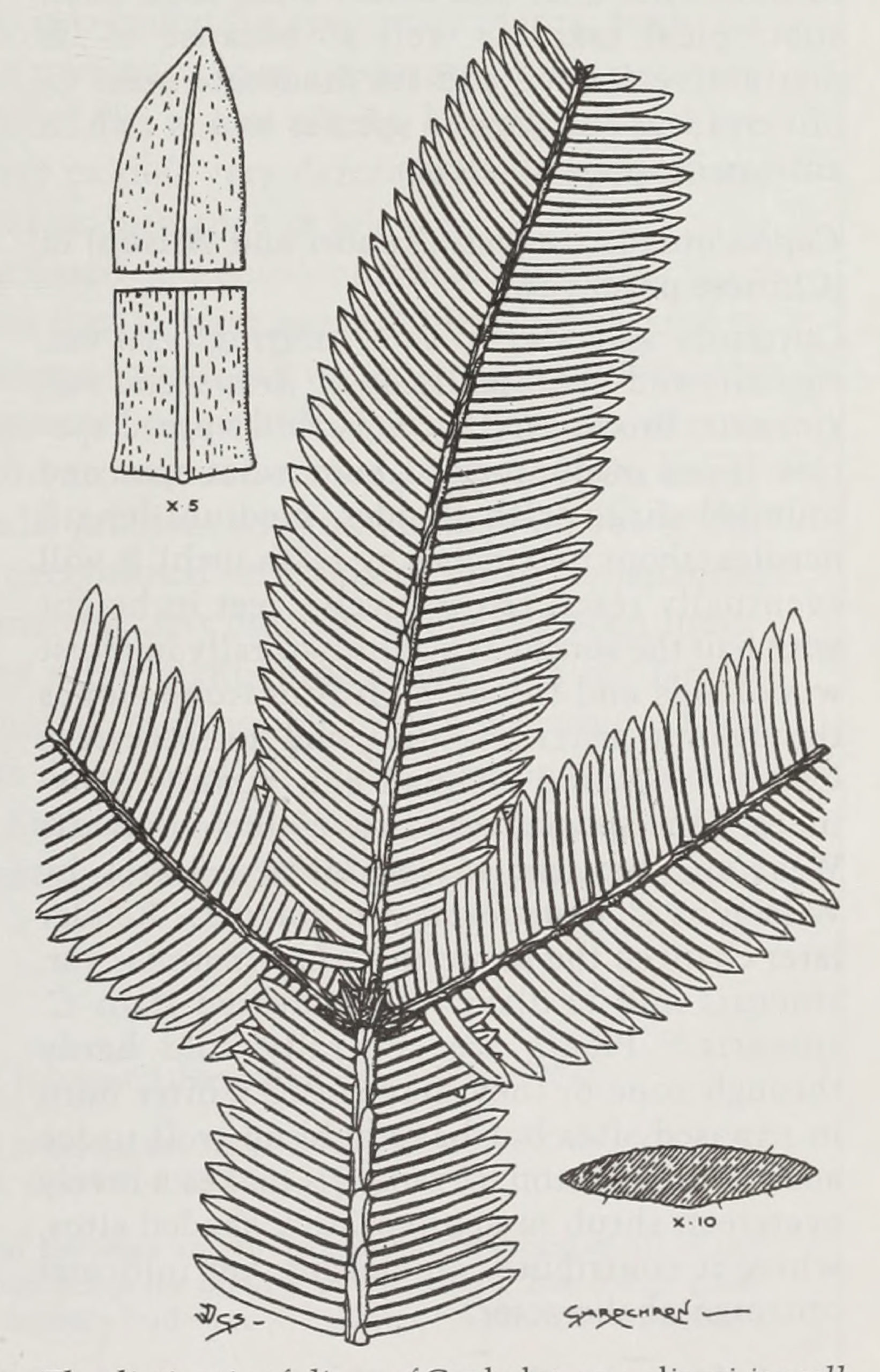
The foliage of this species is among the most distinctive of the genus with short, broad needles (one or one-and-a-half inch by 1/6th inch) arranged in two militarily precise, nearly overlapping ranks. The needles are pectinate, that is, arranged “like the teeth of a comb”;29 this trait remains distinctive even on diverse herbarium specimens. C. oliveri is a large shrub or small tree reaching ten to fifteen feet. It is sometimes found in drier, cooler areas than the other subtropical species of China, but is generally found growing at middle elevations in central, south central, and southwestern China, eastern India, and northern Vietnam. It was once in cultivation at Kew but is no longer listed in their inventory. It is not in cultivation in this country, but it might be a useful horticultural species thanks to its occurrence in somewhat drier and colder areas than other subtropical taxa, as well as because of its distinctive foliage and its moderate size. C. oliveri is an endangered species and is rich in anticarcinogenic alkaloids.
Cephalotaxus sinensis (Rehder and Wilson) Li (Chinese plum yew)
Currently also known as C. harringtonia var. sinensis and historically as C. drupaceae var. sinensis, this is another very widespread species. It is a medium-sized, somewhat open and rounded shrub with slender, medium-length needles (about two inches by 1/8th inch). It will eventually reach ten to twelve feet in height with half the spread. It occurs naturally in moist woodlands and thickets on limestone slopes throughout eastern, central, and northwestern China, including Sichuan and Yunnan provinces. This species was first collected for the West and brought to the United States by Wilson as C. drupaceae var. sinensis; Rehder later changed the name to C. harringtonia var. sinensis and Li ultimately elevated it to C. sinensis.30 Plants are generally cold hardy through zone 6; they may suffer winter burn in exposed sites but have held up well to ice and snow in Boston. C. sinensis makes a lovely evergreen shrub in appropriately shaded sites, where it contributes an elegant, yet informal ornamental character.
Cephalotaxus wilsoniana Hayata (Wilson plum yew, Taiwan plum yew)
This species is endemic to Taiwan, being widely but sparsely distributed in diverse woodlands at middle elevations.31 It is a medium-sized tree, growing to thirty feet with pendant branches. Its needles tend to be slender and of moderate length (about two inches by 1/8th inch). In the United States, it is likely be cold hardy into zone 8. C. wilsoniana is in cultivation at Kew and the Royal Botanic Garden at Edinburgh, but it is not in this country.
Propagation
My work with four of the most hardy species and several cultivars indicates that similar propagation techniques are likely to apply to all Cephalotaxus.32 Propagation from seed or cuttings is quite a long process. Seed gives best germination after ten to twelve weeks of cold stratification and after removal of the fleshy seed coat (which, unlike the similarly constructed Ginkgo, is usually only very slightly malodorous). Seeds that have overwintered outdoors under the mother plants give reasonable germination results as well. Even in a warm greenhouse, seedlings take a worrisome length of time to completely emerge, and it is particularly important to maintain consistently moderate moisture during this period. One is tempted to conjecture that this slow seedling emergence may contribute to the apparently low regeneration rate of Cephalotaxus in the wild.33
Propagation from stem cuttings is not difficult, but it too is slow. In the northeastern United States, four- to six-inch stem cuttings can be successfully rooted throughout the year once the spring flush has been completed and foliage has hardened off somewhat (between July and March). In the southeastern United States, cuttings root best when taken during fall or winter (October to February), avoiding the peak heat of the summer. Stem cuttings will root even without rooting hormones, but moderate concentrations result in slightly larger, fuller root systems. With bottom heat, cuttings generally take about four months to develop a viable root system, although they can take as long as six months in low light. Heavily flowering branches from male plants should be avoided since profuse flowering competes with developing roots, and male flowers are a haven for fungal spores. Flowers on female shoots, on the other hand, have little effect on rooting and do not cause fungal problems. Informal observations in the eastern United States indicate that terminal cuttings are slower to root than lateral ones, sometimes needing an additional two to four weeks; these will, however, result in plants with upright growth. Lateral cuttings, while quicker to root, result in plants with prostrate growth, at least for a number of years. For some as yet unexplained reason, ’Duke Gardens’ has been more difficult to root than other cultivars.
The only challenge in propagating this genus is the degree of patience required. It would be worth experimenting with fog systems to see if they might hasten the process of rooting. Janick et al. reported success with micropropagation of C. harringtoma,34 but to the best of my knowledge no one has yet applied the technique on a commercial scale.
Cephalotaxus at the Arnold Arboretum
The Arnold Arboretum has had a long and significant relationship with Cephalotaxus, having been among the first to collect and cultivate the genus in this country. Several men made important collections of Cephalotaxus for the Arboretum, among them Frank Meyer, William Purdom, Joseph Rock, and Charles Sargent; however, the many collections made by E. H. Wilson included some of the most interesting. In Japan, Wilson collected C. harringtonia, C. harringtonia var. nana, and C. koreana. In China, he collected C. fortunei, C. harringtonia, and C. oliveri, and was the first Westerner to collect what would eventually be named C. sinensis. Throughout his collecting years he consistently expressed an interest in the genus. Both Wilson and Alfred Rehder worked on describing and naming the genus over many years.35
While none of the Wilson-era accessions or their progeny survive at the Arnold, the Arboretum remains actively interested in Cephalotaxus, and its living collections are home to one of the country’s most diverse collections of source-documented, wild-collected germplasm. Among others, Stephen Spongberg has collected material in China, Japan, and Korea. The most recent collections of Cephalotaxus for the Arboretum were made by Peter Del Tredici in China last year. We are especially pleased to have new germplasm of C. sinensis collected by Peter from the northerly portion of its range, which may offer improved cold hardiness and winter performance in the winter landscape.
Citation: Tripp, K. E. 1995. Cephalotaxus: The plum yews. Arnoldia, 55(1): 25–39.
Cephalotaxus was once an integral part of the prehistoric, indigenous flora of both North America and Asia. This genus has long since disappeared in North America and is now seriously endangered in Asia, yet plum yews are among the most interesting, beautiful, and useful of evergreen conifers. Cephalotaxus warrants increased study and conservation—with respect for its importance as both a wild and cultivated conifer.
Acknowledgments
The author gratefully acknowledges Shiu-ying Hu for translation and interpretation of texts from relevant Chmese systematic references, and sincerely thanks Jack Alexander, Allen J. Coombes, Peter Del Tredici, Michael Dirr, Aljos Farjon, Martin Gardner, Richard Hartlage, Andrew Knoll, Gary Koller, Cynthia Osman, M.D., Stephen Spongberg, Chris Strand, Benito Tan, Tom Ward, and Elizabeth Wheeler for critical discussion, useful information, or helpful suggestions.
Notes
1. E H. Wilson, The Conifers and Taxads of Japan (Cambridge: Publications of the Arnold Arboretum, University Press, 1916), v-viri, 6-9.
2. F. von Siebold and J G. Zuccanm, Flora Japonica (1835).
3. K. Fu, “A study on the genus Cephalotaxus Sieb. et Zucc.,” Acta Phytotaxonomica Sinica (1984~ 22(4): 277-288.
4. T. Buchholz, “Generic and subgeneric distribution of the coniferales,” Botanical Gazette ( 1948) 110( 1): 80-91; W. C. Cheng, Sylva Sinica /Beijing: Editorial Commitee of Flora of Woody Plants of China, 1983), ), 379-385; W. C Cheng, L. K. Fu, and C. S. Chao, “Cephalotaxaceae, Cephalotaxus, ” Flora Reipublicae Popularis Sinicae, tomus 7 (Beijing: Science Press, 1978), 422-436; H. H Hu, “Distribution of taxads and conifers in China,” Proc 5 Pacif Sci Congr (1934~ 4: 3273-3288; S. Y. Hu, “Cephalotaxaceae,” m “Notes on the Flora of China IV,” Taiwania ( 1964~ 10: 13-62, 25-31; S. C. Lee, “Distribution of Woody Plants of China,” Taiwania (1963) 9: 11-21; T. B. Lee (Tchang Bok Yi), Illustrated Flora of Korea (Seoul: Hyangmunsa, 1979), 58; H. L. Li., Woody Flora of Taiwan (Narbeth, PA: Livingston, 1963), 38-39; H. L. Li, “New species and varieties in Cephalotaxus, ” Lloydia (1953) 16(3): 162-164, H. L. Li, “Present distribution and habitats of the conifers and taxads,” Evolution (1953) 7: 245-261; J. Ohwi, Flora of Japan (Washington, D.C.: Smithsonian Institution, 1965), 111; A. Steward, Manual of Vascular Plants of the Lower Yangtze Valley of China (Corvallis: Oregon State College, 1958), 61-62.
5. Florin, “The distribution of conifer and taxad genera in time and space,” Acta Horti Bergiana (1963) 20(4): 121-326.
6. See, for example, A. Rehder and E. H. Wilson, “Cephalotaxus,” Plantae Wilsonianae, vol. II, ed. C. S. Sargent (Cambridge: Harvard University Press, 1916), 3-6. The exact position of Cephalotaxus has been argued back and forth since Neger placed it as a single genus in its own family in 1907 (Die Nadelholzer (Koniferen) und ubrigen Gymnospermen, Leipzig), which was contrary to A. W. Eichler’s original placement within the Taxaceae (“Coniferae” in A Engler and K. Prantl’s Die Naturlichen Pflanzenfamihen, Leipzig, 1889).
7. Significant differences m the embryogeny and development of Cephalotaxus from the taxads and other conifers were reported by J. T. Buchholz (“The embryogeny of Cephalotaxus Fortunes,” Bulletin of the Torrey Botanical Club [1925] 52[6]: 311-322) but were most definitively elucidated by H. Singh (“The life history and systematic position of Cephalotaxus drupaceae et Zucc.,” Phytomorphology [1961] 11: 153-197), whose work m this area has remained a standard reference for modern authors treating the group.
8. As, for example, m C. N. Page’s “Cephalotaxaceae,” The Families and Genera of Vascular Plants. I Ptendophytes and Gymnosperms, ed K. U. Kramer and P. S. Green (Berlin & NY: Springer-Verlag, 1990), 299-302; and in The New Royal Horticultural Society Dictionary of Gardening (New York: Stockton Press, 1992), 569.
9. R. Sporne, The Morphology of Gymnosperms (U.K.: Hutchmson, 1965).
10. Page, op. cit.
11. Singh, op. cit.
12. Farjon, C. Page, and N. Schellevis, “A preliminary world list of threatened conifer taxa,” Biodiversity and Conservation (1993) 2: 304-326.
13. Huang, “Cephalotaxus mannu Hook. f.,” China Plant Red Data Book—Rare and Endangered Plants, vol. l, ed. L. K. Fu and J. M. Jm (Beijing. Science Press, 1992), 24-25.
14. Throughout the Cephalotaxus literature, both old and new, there are recurring references to destruction of its habitat due to pressure from humans-both from general activity, like forestry, and from harvesting of the Cephalotaxus itself for various purposes. E. H. Wilson noted the use of the seed as an oil source m Japan (op. cit., p. 7). Barry Yinger, after several trips to Korea, reported the general destruction of populations of koreana by clearcutting (“Notes on Cephalotaxus, the plum yew,” Bull American Conifer Society [1989] 6(3): 57-59). In a 1988 publication, Zou Shou-qing reported that the forest cover of Xishuangbanna prefecture was cut from 60% to 33% over the prior twenty years and lists C. oliveri as one of the endangered relict species there (“The vulnerable and endangered plants of Xishuangbanna prefecture, Yunnan province, China” Arnoldia [1988] 48(2): 3-7) Both Cephalotaxus species listed in the China Plant Red Data Book are reported to be threatened by lumbering, and C mannu is also reported to be endangered by harvesting for use as a medicinal herb (see Q. Huang, “Cephalotaxus mannu Hook. f,” and Z. C. Luo et al., “Cephalotaxus oliveri Mast.” in the China Plant Red Data Book, 24-27).
15. The importance of Cephalotaxus has expanded beyond horticulture to include potential use as a source of anticancer compounds found m its tissues (C. R. Smith, R. G. Powell and K. L. Mikolajczak, “The genus Cephalotaxus, source of homoharringtonine and related anticancer alkaloids,” Cancer Treatment Rpt [1976] 60: 1157-1170). The ester alkaloids cephalotaxme, harringtonine, and allied chemicals have shown significant antitumor activity in a number of in vitro studies, and there are recent reports of phase I chemical trials (pharmacokinetic) (see D. M. Graifer et al., “Effect of alkaloids of the Cephalotaxus group on the elongation of the polypeptide chain on human ribosomes,” Molecular Biology [1991] 24(G/: 1344- 1350), and phase II clinical trials (therapeutic) on human subjects as well (see C. T. Tan et al., in Cancer Treatment Reports [1987] 71 1245-48, cited m E. R. Wickremesinhe and R. Arteca, “Establishment of fast-growingg callus and root cultures of Cephalotaxus harringtonia,” Plant Cell Reports 12 [1993], 80-83/. Other recent publications indicate that progress has been made in development of separation (see D. G. Cai et al., “Semipreparative separation of alkaloids from Cephalotaxus fortunei Hook f. by high-speed countercurrent chromatography,” Journal Liquid Chromatography [ 1992] 15: 2873-2881) and synthetic production systems for cephalotaxmes and harringtonines (see T. P. Burkholder and P. L. Fuchs, “Total synthesis of the Cephalotaxus alkaloids dl-cephalotaxme, dl-11-hydroxycephalotaxine, and dl-drupacme,” Journal American Chemical Society [ 1990] 112: 9601-9613; and M. Ikeda et al., “Synthetic studies on Cephalotaxus A synthesis of (=)-cephalotaxme,” Chemical and Pharmaceutical Bulletin [1993] 41(2): 276-281), as well as improvements on Cephalotaxus tissue proliferation techniques (see P. J. Westgate et al., “Approximation of continuous growth of Cephalotaxus harringtonia plant cell cultures using fed-batch operation,” Biotechnology and Bioengineering [1991] 38: 241- 246; and E. R. Wickremesinhe and R Arteca, op. cit.)
16. P Chu, “A study of the alkaloids in Cephalotaxus and their bearing on the chemotaxonomic problems of the genus,” Acta Phytotaxonomica Sinica (1979) 17(4): 7-20.
17. The primary conifer references, such as Krussman’s 1976 (1984 translation) Manual of Cultivated Conifers (Portland, OR: Timber Press, 66-69), pl. 63, and Humphrey Welch’s 1990 The Conifer Manual, vol. I (Netherlands: Kluwer Academic) 191-194, rely on these often tenuous, morphological characters to distinguish Cephalotaxus
18. A specific example of such a tenuous morphological trait used to distinguish a taxon is the “V” outline supposedly created by the foliage of harringtonia var. drupaceae, which has been cited by many prominent references to separate C harringtonia var. drupaceae from other Cephalotaxus In reality, this trait can be seen on many plants of Cephalotaxus regardless of species-see, for example, W. J. Bean, Trees and Shrubs Hardy in the British Isles (England: John Murray, 1950), 405-406; P Den Ouden and B. K. Boom, Manual of Cultivated Conifers (The Hague: Martinus Nijhoff, 1965), 65-69; Hillier Nurseries, The Hillier Manual of Trees and Shrubs, 6th ed. (Devon, England: David and Charles, 1991), 584- 585, 677, 679; G Krussman, op. cit ; J Lewis, “Cephalotaxaceae,” The European Garden Flora, vol. I, ed. S. M. Walters et al. (London: Cambridge University Press, 1986), 73-74; and H. J. Welch, op. cit. 1
19. Y. Hu, “The Metasequoia flora and its phytogeographic significance,” Journal of the Arnold Arboretum (1980) 61: 41-94.
20. Interestingly, Fortune collected this species and sent it back to the USDA as part of a shipment of material collected on an expedition m search of the best forms of tea plants (Camellia sinensis) (see R. Gardener, “Robert Fortune and the cultivation of tea in the United States,” Arnoldia (1971) 31 (1 ): 1-18) This was a fortuitous opportunity to include Cephalotaxus as part of the collections.
21. In fact, oliveri was originally confounded with C griffithii Oliver’s 1890 illustration of C. griffithii was C oliveri (see D. Oliver, “Cephalotaxus griffithii,” ” p1.1933, Hooker’s Icones Plantarum, vol. X, pt. I, 3rd series, J. D. Hooker, 1890) This was later clarified by Masters (see The Gardener’s Chronicle [1903] 850: 226-228).
22. D. Hooker, Flora of British India, vol. V (London, 1890), 647-648.
23. As reported by J. Ohwi in the 1965 Flora of Japan (Washington, D. C.: Smithsonian Institution), p. Ill, and as observed by S. Spongberg when traveling m Japan.
24. For notes on collections made during the Spongberg and Weaver expedition to Japan and Korea, see S A. Spongberg, “Korean Adventure,” Arnoldia (1978) 38(4): 132-152, and S. A. Spongberg and R. E. Weaver, Jr., “Collecting expedition to Japan and Korea,” Arnoldia ( 1978) 38 (1) 28-31
25. ‘Korean Gold’ was described in C. Hahn and B. Yinger, “Cultivars of Japanese plants at Brookside Gardens,” Arnoldia (1983) 43/4/. 3-19.
26. Hillier Nurseries, op. cit.
27. See, for example, the primary reference, W. C Cheng et al., op. cit.
28. 1523 m J. D. Hooker’s “Cephalotaxus mannu” (op. cit., 1890) shows the elegant character of this foliage beautifully. Hooker remarks in the accompanying text on C mannu, “A very distinct species … but so like Taxus baccata as to be easily mistaken for it.” Recall the elegant, gracefully tapering outline of English yew, and you will understand the comparison.
29. T. Masters, op. cit.
30. Alfred Rehder in the 1941 article, “New species, varieties and combinations from the herbarium and the collections of the Arnold Arboretum” (Journal of the Arnold Arboretum 22: 569-571) changed the name of what had been called C drupaceae to harringtonia (he concluded that harringtonia was the older of the two specific epithets), and hence, all of the included botanical varieties became C. harringtonia Subsequently, H. L. Li, m his 1953 article, “New species and varieties in Cephalotaxus, ” elevated it to the species C sinensis.
31. H L. Li, 1963, op. cit.
32. For further information on propagation of Cephalotaxus, see M A. Dirr and C. W. Heuser, Jr., The Reference Manual of Woody Plant Propagation (Athens, GA: Varsity Press, 1987/, 104, A. Fordham and L. Spraker, “Propagation manual of selected gymnosperms,” Arnoldia (1977) 37 (1) /: 48; and J. A. Young and C. G Young, Seeds of Woody Plants in North America (Portland, OR: Dioscorides Press, 1992), 93.
33. Huang, op. cit., cites a naturally low pollination rate, and Luo et al., op. cit, report infrequent regeneration, but neither source cites other work m support of these statements. It is possible that a combination of dioecious reproductive biology, relatively slow seed maturation and germination, seed predation by birds and mammals, increasingly sparse distribution of mature plants, and general destruction of habitat favorable for seedling survival and development, leads to the reported infrequent regeneration of Cephalotaxus
34. Janick et al., “Micropropagation of Cephalotaxus harringtonia,” HortScience (1994) 29 (2): 120-122.
35. For details of taxa collected on specific expeditions and early taxonomic commentary on various taxa, see A. Rehder, “Enumeration of the ligneous plants of northern China,” Journal of the Arnold Arboretum (1923) 4 (3): 117-128; A. Rehder, “New species, vane ties and combinations from the herbarium and the collections of the Arnold Arboretum,” Journal of the Arnold Arboretum (1923) 4. 107; A. Rehder and E H. Wilson, “Enumeration of ligneous plants collected by J. F Rock on the Arnold Arboretum expedition to northwestern Chma and northeastern Tibet,” Journal of the Arnold Arboretum (1928) 9: 5-20; A. Rehder and E. H. Wilson, “Cephalotaxus“; E H. Wilson, “The taxads and conifers of Yunnan,” Journal of the Arnold Arboretum (1926) 7: 39-68, and E. H. Wilson, The Conifers and Taxads of Japan, op. cit.
Kim E. Tripp is a Putnam Fellow at the Arnold Arboretum, using the living collections for research, teaching, and writing.
From “free” to “friend”…
Established in 1911 as the Bulletin of Popular Information, Arnoldia has long been a definitive forum for conversations about temperate woody plants and their landscapes. In 2022, we rolled out a new vision for the magazine as a vigorous forum for tales of plant exploration, behind-the-scenes glimpses of botanical research, and deep dives into the history of gardens, landscapes, and science. The new Arnoldia includes poetry, visual art, and literary essays, following the human imagination wherever it entangles with trees.
It takes resources to gather and nurture these new voices, and we depend on the support of our member-subscribers to make it possible. But membership means more: by becoming a member of the Arnold Arboretum, you help to keep our collection vibrant and our research and educational mission active. Through the pages of Arnoldia, you can take part in the life of this free-to-all landscape whether you live next door or an ocean away.