Like many of his colleagues, structural biologist David Goodsell uses computation to visualize how molecules interact in living organisms. Distinctively, Goodsell also makes paintings of these interactions—“mesoscale molecular landscapes,” as he describes them—depicting the realm where molecules come together at nanometer scale to build living cells and tissues. Phenomena at these scales aren’t truly “seeable” in the usual sense, as they’re smaller than the wavelengths of visible light our eyes use to resolve images. Scientists have developed diverse ways of seeing and knowing at molecular scale: electron microscopy, crystallography, spectroscopy, chemical and genetic assay and, increasingly, computational modeling. These methods interact with molecules in different ways, at different scales, yielding a wide array of data, which Goodsell interprets and synthesizes in paintings like the ones you see here.
In the worlds that trees make, this mesoscale matters. It’s where the chemical exchanges of root tissues and endomycorrhizal fungi take place, the garden where the complex molecules useful to humans and other animals are nurtured. As in Darwin’s image of the tangled bank, Goodsell’s botanical mesoscale is a landscape of “elaborately constructed forms, so different from each other, and dependent upon each other in so complex a manner.” His images here take us deep within living plants, depicting the manufacture of cellulose comprising plant cell walls and the quantum-driven exchange of energies that happens with photosynthesis in the grana of chloroplasts.
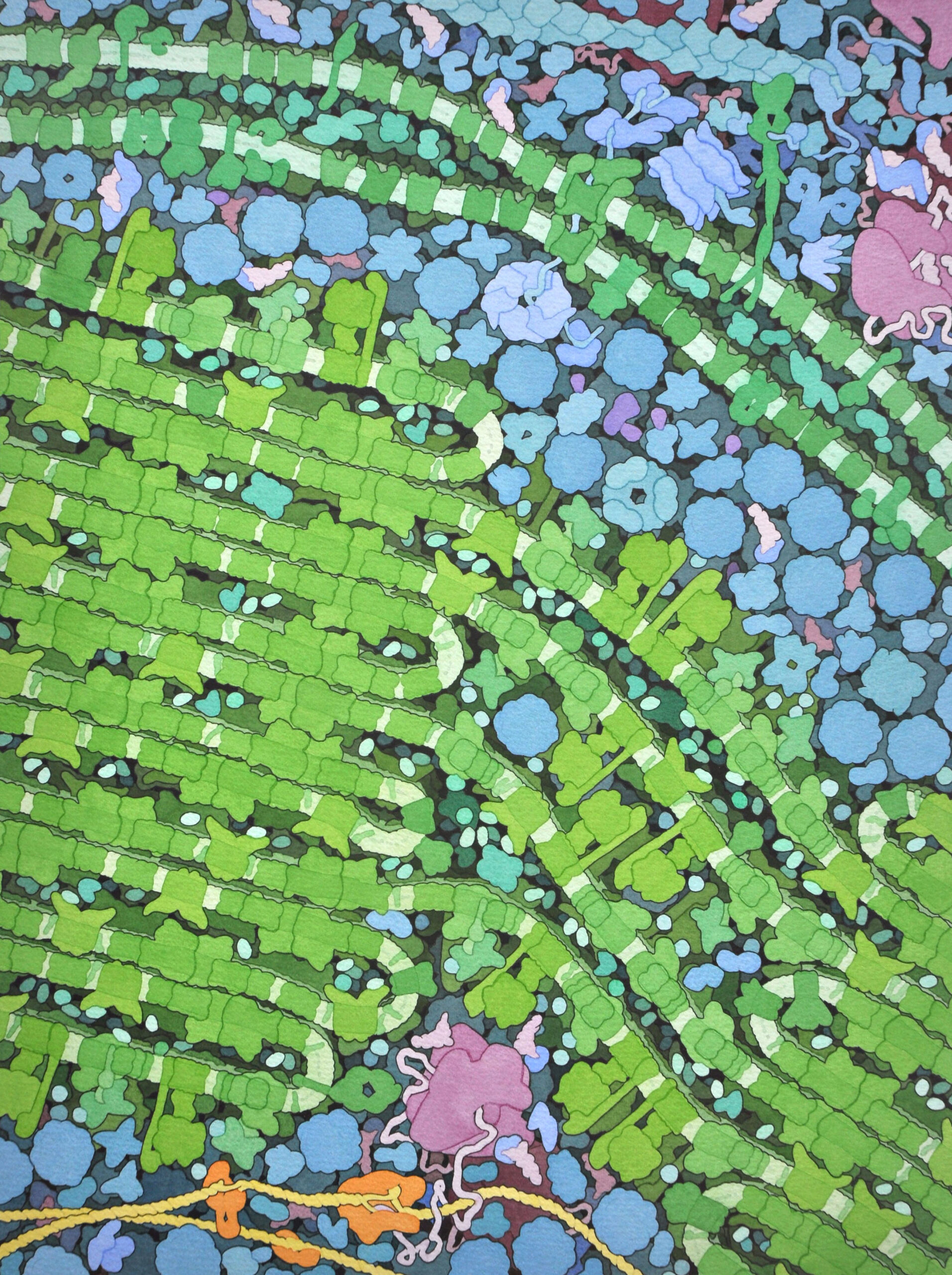
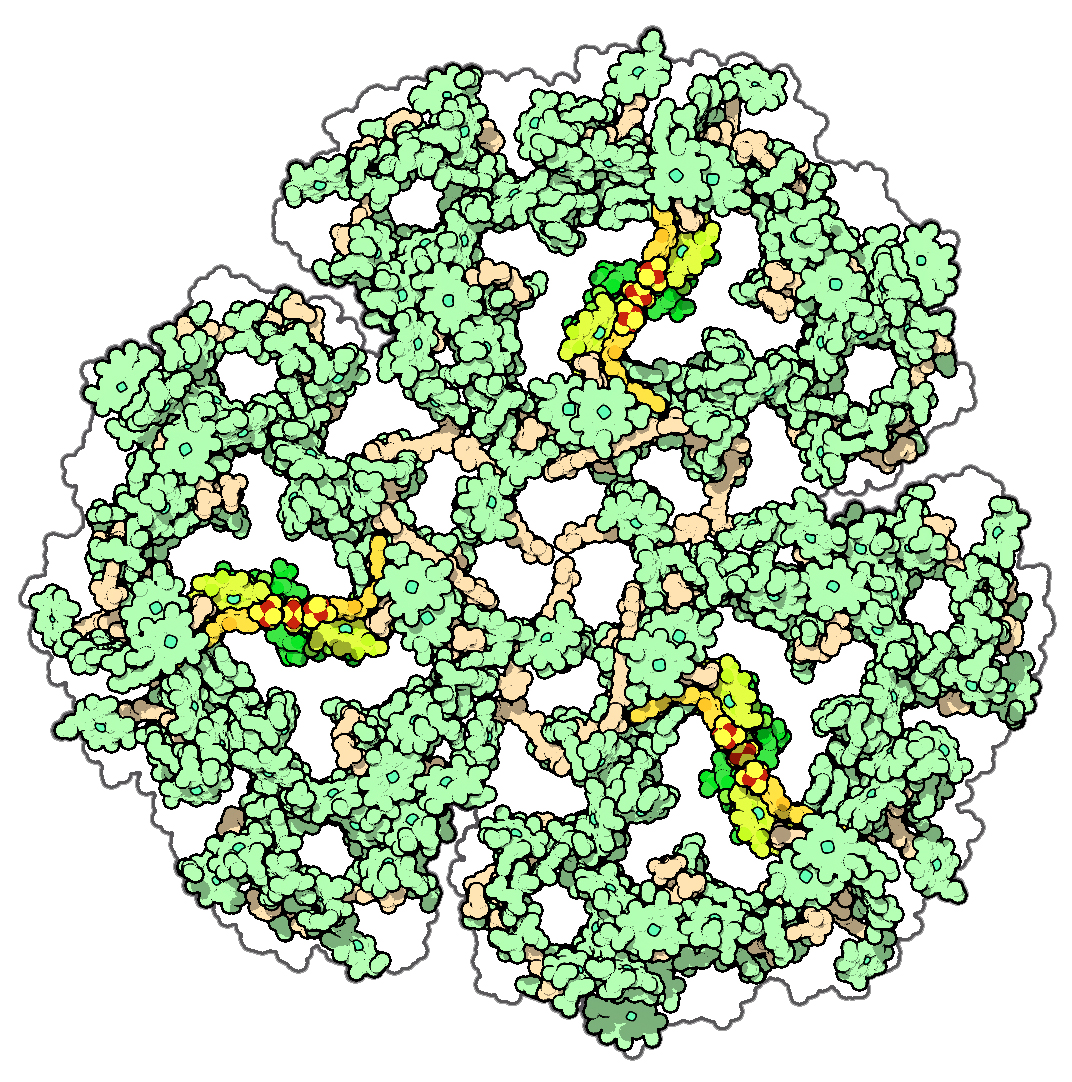
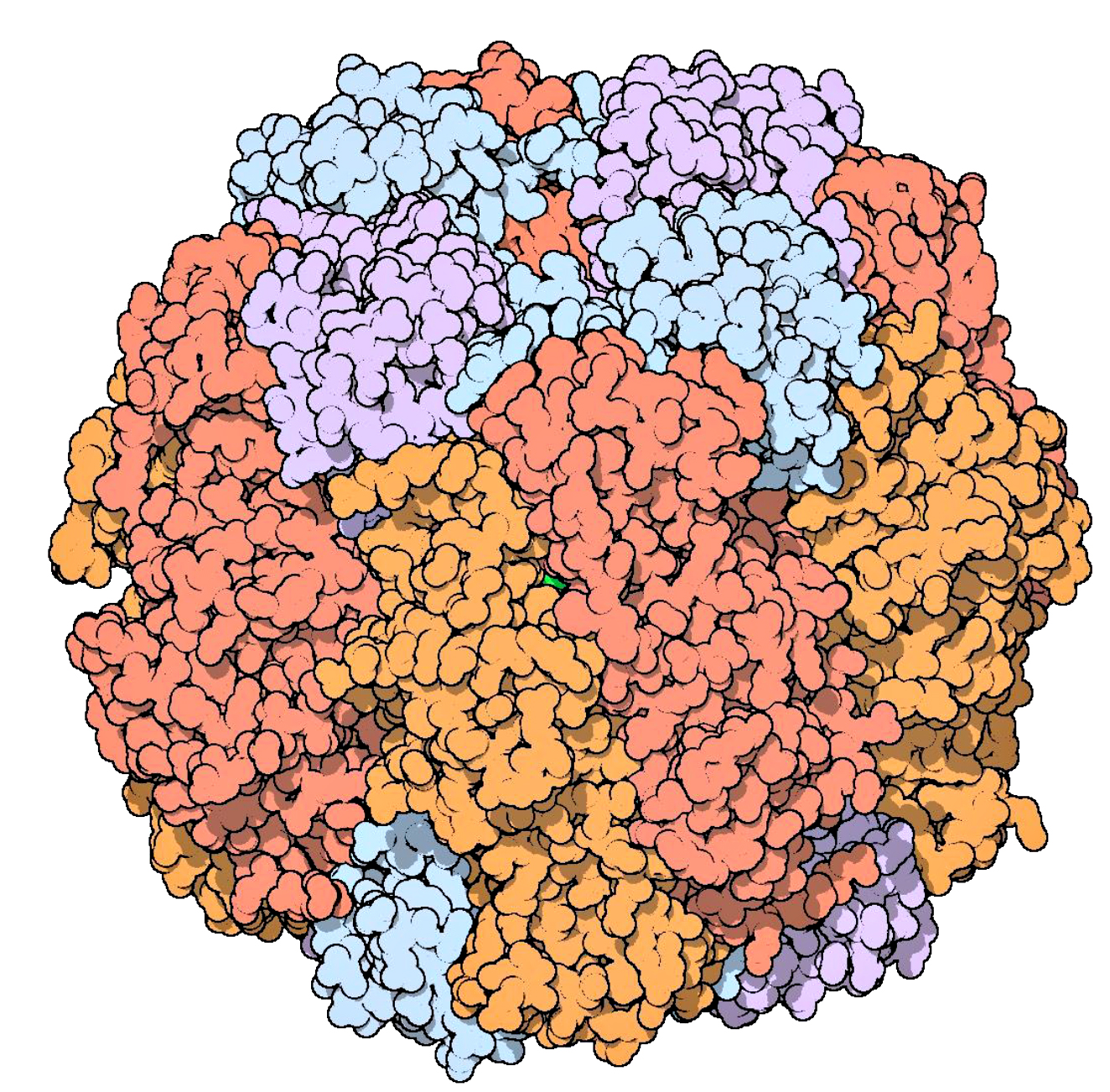
The words “cell” and “cellulose” share a common origin. The material was named for the structures they form, now called “cells”—the term Robert Hooke coined in his Micrographia (1665) to name the smallest structures he could see through his microscope. In plants, the chief material and structural ingredient of this intercellular matrix is cellulose, making it the most abundant biopolymer in the world. Perhaps only limestone outstrips its sheer magnitude as a material produced by living organisms.
The cell wall is very far from being a simple, monolithic slab of cellulose, however. In the painting below, the exterior of the cell lies beyond the top margin of the image, with the cell membrane and the jumbled systems of the cell’s interior in greens and blues at bottom. The boundary between membrane and wall is lively, productive, tensed with the straining forces of polymer production. The membrane is studded with proteins able to transmit molecules into and out of the cell. It is also the substrate where the synthesis of cellulose unspools. Regularly spaced here, streams of the polymer emerge among the tangled proteins and carbohydrates of the cell wall from collars of cellulose synthase, which fuse them together like strands woven in a rope loom. The cellulose synthase structures are moved into place and supported by microtubules—cylindrical polymer beams, which act as a transit system and structural element, like girders and tramways threading through the cytoplasm. Here, a microtubule is the basis of a molecular assembly line, fixing and supporting the cellulose synthase arrays. As the strands of cellulose spout forth, they interweave with pectins, xyloglucans, and other branching, long-chain carbohydrates—including lignin, which in trees combines with cellulose to make wood.

David Goodsell is Professor of Computational Biology at the Scripps Research Institute, and Research Professor at Rutgers University, where he is Scientific Outreach Lead at the RCSB Protein Data Bank.
